This article has been corrected. See "Erratum: Addition of a Co-Author" in Volume 28 on page 1260.
Abstract
The first case of human cryptosporidiosis was reported in Korea in 1995; however, an outbreak of Cryptosporidium has not been reported in Korea until now. This paper describes the first outbreak of cryptosporidiosis in Korea. On May 24, 2012, a local public health center filed a report on 126 residents with gastrointestinal symptoms in an old apartment complex in Seoul. Epidemiological investigations were implemented on 125 of the 126 patients. The patients were reported continuously over a period of 22 days. Diarrhea was the most common clinical symptom, and lasted for 5 days on average. The tap water was the only common exposure of the patients. During the environmental investigation it was discovered that the water and septic tanks were situated closely and that the waste water pipes were corroded where they passed over the water pipes. Cryptosporidium parvum was detected in 3 of the 7 stool specimens by PCR-RFLP. A number of Cryptosporidium oocysts were also detected in the water specimens from the water tank. In conclusion, Cryptosporidium parvum was the key causal pathogen of this outbreak. It is presumed that the tap water was contaminated by a sewage leak from the aged pipelines.
Cryptosporidium is a minute coccidian parasite with worldwide distribution. Two species of Cryptosporidium, C. hominis and C. parvum, are the major micro-organisms of human infection (1, 2). Recently, the outbreaks of cryptosporidiosis have increased due to increase in recreational water use (3-5). The first human infection of Cryptosporidium was reported in Korea in 1995 (6) and since then, epidemiologic studies were carried out on a diverse range of regions (7-11). Although a positive rate of Cryptosporidium oocysts in human less than 3% was reported in most of the regions, some regions reported a very high positive rate of more than 40%. The livestock in such regions also displayed high level of positive rates. Therefore, it is possible to presume that zoonotic transmission is the most important cause of Cryptosporidium infection in Korea. However, in spite of such high level of positive rates in specific regions, an outbreak of Cryptosporidium has not been reported in Korea until now. This study is reporting the first outbreak of Cryptosporidiosis in Korea. This outbreak could be deemed to be highly significant in that it has displayed unique epidemiologic characteristics irrelevant to the usage of recreational water or the zoonotic transmission.
This study is a case series study. On May 24, 2012, a local public health center filed a report on 126 residents with diarrhea in an old apartment complex in Seoul, Korea. An epidemiological investigation was immediately undertaken by a Seoul Metropolitan Epidemic Intelligence Service (EIS) officer in Seoul along with infectious disease inspectors from the local Public Health Center. Korea Centers for Disease Control and Prevention (KCDC) was consulted on this epidemiological investigation. Clinical specimens including stool specimens and rectal smear samples were taken for microbiological examination during the epidemiological investigation. Water specimens from the water tank in the apartment complex and the adjacent building were collected by the Waterworks Research Institute of the Seoul Metropolitan Government.
The case was defined as the residents of the apartment complex, who had frequently suffered from watery diarrhea more than twice, or two of the following symptoms, namely, watery diarrhea, abdomen pain, fever and vomiting in May 2012.
Data were collected by using the structured case report form during the epidemiologic investigation. The case report form included the following variables: the demographic features such as sex, age and address, the common exposures, clinical features and date of onset.
The clinical specimens were primarily examined by the Seoul Research Institute of Public Health and Environment for the bacteriological and virological examination as the Epidemiologic Investigation Guideline for Food and Waterborne Diseases from KCDC (12). The stool specimens were subsequently examined by the Korea National Institute of Health for parasitological examination. The protozoa detection was undertaken by polymerase chain reaction (PCR) and polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) (13). PCR was performed in 20 µL reactions containing 4 µL of DNA template, 10 pM of each primer, 1.25 U of Ex Taq DNA polymerase (iNtRON Biotechnology, Seongnam, Korea), 1.5 mM MgCl2, and 0.2 mM of each dNTP. The primers that were used were CP2-415F (upstream), 5'-CCCACGCGAAGTTGAAGTAAC-3' and CP2-415-R (downstream), 5'-CTTAGGTTGCTTGCTTGGAGTTGG-3'. The amplified size was 415 bp fragment. The PCR conditions used for first-step PCR were 1 cycle of 94℃ for 5 min, 35 cycles of 94℃ for 30 sec, 53℃ for 30 sec, and 72℃ for 90 sec, and final extension of 1 cycle of 72℃ for 10 min. The nested-PCR was performed using 1 cycle of 94℃ for 5 min, 35 cycles of 94℃ for 30 sec, 55℃ for 30 sec, and 72℃ for 90 sec, and final extension of 1 cycle of 72℃ for 10 min. For the nested-PCR, the primers were 5'-CAACCAGAAGTTGAGGTT-3' (upstream) and 5'-CTAGTATGCTTCAGACCATGA-3' (downstream). The nested PCR amplified 171 bp fragment. Each PCR product obtained by nested-PCR analysis was purified with a QIAquick Gel Extraction kit (QIAGEN Inc., Valencia, CA, USA) and was digested with restriction enzymes of BsiEI to identification of Cryptosporidium hominis and C. parvum (13). The water specimens were examined by the Waterworks Research Institute of Seoul Metropolitan Government. The analyzed pathogens are listed on Table 1.
The apartment complex, consisting of three buildings, was built in 1977. A total of 228 households (564 people) were residing in the apartment complex at the time of the incident. Total number of patients reported was 126 during the period of epidemiologic investigations with prevalence rate of 22.3% (126/564). Of the 126 patients, epidemiologic investigations were implemented on 125 patients.
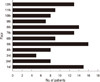
There were more females (55.2%) than the males (44.8%) and the mean age was 46.95 yr. The prevalence rates in each apartment were as follows: 62.1% per one household for building-A, 40.7% for building-B and 63.6% for building-C (Table 2). The patients were reported throughout the entire floors of the apartment (Fig. 1).
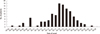
The patient was first found on May 3 and the last patient on May 28. The peak time of the incidence was May 18 (Fig. 2).
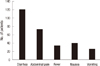
Of the 125 patients, 121 patients suffered from diarrhea, while 73 patients suffered from abdominal pain, 34 patients suffered from fever and 26 patients suffered from vomiting. On average diarrhea was continued for five days (Fig. 3).
As the results of epidemiologic investigations, it was found that there were no abnormalities prior to the outbreak through which all the patients could be commonly exposed to the pathogen. The tap water was the only common source of exposure of the patients to the pathogen. Accordingly, investigation was conducted on the usage of tap water among the patients. All the patients used tap water for domestic purposes including cooking. A total of 116 out of 125 patients used tap water for drinking while the remaining 9 did not. Of the 116 patients who used tap water for drinking, 50 used water purifier, 60 boiled the tap water, and 6 boiled the water from the water purifier. There was no patient who drank tap water without any kind of treatments.
The water tank that stored the tap water was buried underground in the vacant lot at the center of the apartment complex. Septic tanks were situated within the distance of 3meters in its surrounding. The external wall of the underground water tank was constructed with concrete and had no crack. The upper portion of the underground water tank was covered with manhole. Structural abnormality of the pipeline was observed during the investigation of the pipeline structure. The wastepipes for the septic tank crossed over the water pipes for the water tank. These pipes were aged and worn out (Fig. 4).
During the epidemiological investigation, 20 rectal smear samples and six stool specimens were collected from the residents suffering from severe diarrhea. Additionally, nine rectal smear samples and one stool specimen were taken from the residents who started diarrhea after the investigation. Staphylococcus aureus was detected in two specimens among 29 rectal smear samples and seven stool specimens, and C. parvum had been detected in 3 of the 7 stool specimens by PCR-RFLP (Table 3 and Fig. 5). Also, a number of bacteria and C. parvum oocysts were detected in the tap water of underground water tank. On the other hand, there was no other pathogen detected in the tap water of the nearby buildings (Table 4).
Due to the suspicion of tap water as the source of infection, the supply of tap water was cut immediately. During this period of stoppage in the supply of tap water, the pipeline structure was corrected and the water tank and pipelines were disinfected. Cutting off of the tap water supply was maintained until no Cryptosporidium oocysts were detected through continuous water test, and it took approximately 4 weeks before the Cryptosporidium oocysts were found no more. There was no report of new patient since the cutting off of the tap water supply. Although there was a report of patients with recurring symptoms during the same period, all the patients recovered without complications. The occurrence of patients was monitored for 5 weeks since the resuming of the tap water supply, and there was neither report of new patients nor patients with recurring symptoms.
Cryptosporidium sp. is diagnosed by identifying organism in intestinal biopsy material, and by microscopically detecting oocysts following modified acid fast stains or fluorescent stains in stool specimens (14-16). Cryptosporidium can also be detected by enzyme immunoassay or PCR (17-20). PCR could be an excellent diagnostic method for Cryptosporidium sp. because PCR does not need highly trained expert and reduces the diagnostic labor time. Moreover, PCR-RFLP could differentiate the two species of Cryptosporidium, such as C. parvum and C. hominis.
In this study, C. parvum was detected by PCR-RFLP. Eight patients who recovered at the time of investigation refused the stool examination, and 22 among 29 patients, who had clinical symptoms during epidemiologic investigation only consented to taking of rectal smear samples, and denied taking of stool specimens. C. parvum was detected in 3 of the 7 stool specimens in this study. In addition, a number of C. parvum oocysts were detected in the tap water from the water tank. In the guidelines for confirmation of food-borne disease outbreak (21, 22), Cryptosporidium spp. can be confirmed as the causal pathogen for the outbreak if Cryptosporidium was detected in stool specimens or in small bowel biopsy of two or more ill persons, or if pathogen is detected in the epidemiologically implicated food. Although small number of stool specimens was examined, C. parvum could be detected in more than two patients and in the tap water, which was epidemiologically implicated. Therefore, it provided sufficient ground to decide C. parvum as the cause of the outbreak.
However, there is a limitation in concluding that all 126 reported patients have been infected by C. parvum because a number of different bacteria were detected in the tank water specimens. These bacteria implied stool contamination of the tap water. However, there was no bacterium or virus that identified in the stool specimens and rectal smear samples except Staphylococcus aureus. S. aureus was detected in two rectal smear samples. It was presumed that possibility of S. aureus being the major pathogen for this outbreak was low because the clinical symptoms in the patients differed from those caused by S. aureus. Based on the laboratory results, it was concluded that C. parvum was the key causal pathogen of this outbreak.
The numbers of water borne and food borne disease outbreaks in Korea were 355 in 2008, 227 in 2009, 254 in 2010, 236 in 2011 and 288 in 2012 respectively. Majority of outbreaks were due to infection at ordinary restaurants. In the large-scale outbreaks with more than 100 people being affected, the main cause was consumption of contaminated food at group canteen, including schools. These outbreaks lasted less than 2 weeks. As the results of internal data review on the water borne and food borne disease outbreaks that reported to the Korea Centers for Disease Control and Prevention over the last 5 yr, there has not been any outbreak of waterborne disease like this case with large number of patients with more than 100 patients being affected over a period of more than 3 weeks.
Although there has not been any domestic report of the outbreak of cryptosporidiosis until now, there have continually been literary reports on investigations of positive rates of Cryptosporidium oocysts in human in different regions. Low positive rate of 0.5% was reported in Seoul (8), 1.1% in Chungju (9), 1.9% in Cheorwon (7), 2.2% for Chuncheon (8), 0.4% in Haman-gun, and 1.1% for Euryeong-gun (9). However, high positive rate of 10.5% was observed for Jeollanam-do, and while low positive rate of 3.7% was observed for urban regions such as Mokpo and Yeosu. Hwasun-gun displayed high positive rate of 40% (8), which continued to be maintained at the high rate during the investigations conducted afterwards (10). In addition, low positive rate of 1.5% was observed in the island regions of Jeollanam-do (11). Such epidemiologic characteristics of Jeollanam-do were presumed to be the results of the zoonotic transmission because the high rates of Cryptosporidium infection were reported in the livestock in the regions with high positive rates. However, this study was different from the previous ones because the present outbreak was irrelevant with the zoonotic transmission.
The symptoms of cryptosporidiosis develop after an incubation period about 1 week (23). Watery diarrhea is the main symptom with possible accompaniment by abdominal pain, nausea, vomiting, anorexia, fever and reduction in body weight. In general, the symptoms last approximately 5-10 days in immunocompetent individuals (24). Approximately 39% of the patients display biphasic patterns in which symptoms recur within several days to several weeks after the symptoms disappear (24-26). This outbreak also displayed the majority of patients having symptoms of watery diarrhea accompanied by abdominal pain, fever and vomiting. Median duration of illness was about five days. In addition, the patients with recurrence of symptoms were reported in this outbreak. The clinical characteristics of patients in this outbreak coincided with the clinical symptoms of cryptosporidiosis.
The investigation on the usage of tap water was conducted to deduce the transmission route of Cryptosporidium. In spite of the investigation, the transmission route was not exactly identified in this study. However, it was still possible that some of the residents might be exposed to sufficient quantity of Cryptosporidium oocysts that could cause infection through the usage of tap water for cooking and washing. The reason is that Cryptosporidium spp. could cause infection with minute quantity in the range of 10-30 oocysts (27).
In order to find the cause of contamination of the tap water, water specimens from the adjacent buildings were taken for an investigation. These buildings use water that has not passed through the underground water tank, even though the same water supply network is used. No pathogen was detected in the water specimen in these buildings. This indicates that there was no contamination of the tap water prior to being stored in the underground water tank. With the old pipelines of the water tank and the septic tanks and structural abnormalities of pipeline network in the apartment complex, it is presumed that contaminated water was mixed with the water in the underground water tank by leakage of sewage in wastepipe.
There are several limitations in this study. Firstly, the epidemiological investigation to the patients without symptoms was not made. Secondly, the stool examinations from the patients who were already recovered were not conducted. Thirdly, the leakage of pipelines of the underground water tank and septic tank was not confirmed by means of dye tests. Fourthly, the cause of C. parvum contamination of the sewage was not clarified. For this, demographic data including occupation and underlying disease from all residents in the apartment complex should be collected. Because a number of the residents decline to participate in the epidemiological investigation, further evaluation could not be conducted. However, the previous study about the prevalence of Cryptosporidium spp. in the Han-river which passed through Seoul showed that domestic wastewater from the urban region could be a source of Cryptosporidium spp. contamination (28).
In conclusion, the outbreak was due to C. parvum in the contaminated water of underground water tank. It is presumed that the water in the underground water tank was contaminated by sewage in the septic tank through the old pipelines. This report is significant in that it is the first outbreak of cryptosporidiosis in Korea, and occurred in the urban area without the risk factors like recreational water use or livestock infection. Since detection of Cryptosporidium spp. is not a test generally performed in the outbreak of waterborne disease, it can be easily overlooked unless careful attention is paid. If the causal pathogen in the outbreak that occurs in urban regions is not clear, then, it could be helpful to implement tests for the presence of Cryptosporidium species.
Figures and Tables
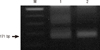 | Fig. 5PCR-RFLP pattern of amplified Cryptosporidium parvum. The amplified DNA by PCR was digested with BsiEI. Lane 1, Marker; Lane 2, Control; Lane 3, Patient stool sample. |
ACKNOWLEDGEMENTS
We express our gratitude to our colleagues in the Seoul RIHE, the Waterworks Research Institute of Seoul Metropolitan Government and Department of Malaria and Parasite Disease in the National Institute of Health for the laboratory examination to detect the pathogen. Also, we thank Dr. Jae-Ran Yu for providing the control DNA of C. parvum.
References
1. Chalmers RM, Davies AP. Minireview: clinical cryptosporidiosis. Exp Parasitol. 2010. 124:138–146.
2. Cama VA, Bern C, Roberts J, Cabrera L, Sterling CR, Ortega Y, Gilman RH, Xiao L. Cryptosporidium species and subtypes and clinical manifestations in children, Peru. Emerg Infect Dis. 2008. 14:1567–1574.
3. Yoder JS, Harral C, Beach MJ. Centers for Disease Control and Prevention (CDC). Cryptosporidiosis surveillance - United States, 2006-2008. MMWR Surveill Summ. 2010. 59:1–14.
4. Takagi M, Toriumi H, Endo T, Yamamoto N, Kuroki T. An outbreak of cryptosporidiosis associated with swimming pools. Kansenshogaku Zasshi. 2008. 82:14–19.
5. Baldursson S, Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks - an update 2004-2010. Water Res. 2011. 45:6603–6614.
6. Kang YK, Lee HK, Kim SW, Chi JG. Cryptosporidiosis in a leukemia child with severe diarrhea. Seoul J Med. 1995. 36:29–34.
7. Seo M, Huh S, Chai JY, Yu JR. An epidemiological survey on Cryptosporidium parvum infection of inhabitants in Chorwon-gun, Kangwon-do. Korean J Parasitol. 2001. 39:201–203.
8. Chai JY, Lee SH, Guk SM, Lee SH. An epidemiological survey of Cryptosporidium parvum infection in randomly selected inhabitants of Seoul and Chollanam-do. Korean J Parasitol. 1996. 34:113–119.
9. Yu JR, Lee JK, Seo M, Kim SI, Sohn WM, Huh S, Choi HY, Kim TS. Prevalence of cryptosporidiosis among the villagers and domestic animals in several rural areas of Korea. Korean J Parasitol. 2004. 42:1–6.
10. Park JH, Guk SM, Han ET, Shin EH, Kim JL, Chil JY. Genotype analysis of Cryptosporidium spp. prevalent in a rural village in Hwasun-gun, Republic of Korea. Korean J Parasitol. 2006. 44:27–33.
11. Park JH, Kim HJ, Guk SM, Shin EH, Kim JL, Rim HJ, Lee SH, Chai JY. A survey of cryptosporidiosis among 2,541 residents of 25 coastal islands in Jeollanam-do (Province), Republic of Korea. Korean J Parasitol. 2006. 44:367–372.
12. Korea Centers for Disease Control and Prevention. Epidemiologic investigation guideline for food and waterborne diseases 2012. 2012. Cheongwon: Division of Epidemic Intelligence Service, Korea Centers for Disease Control and Prevention.
13. Lee SH, Joung M, Yoon S, Choi K, Park WY, Yu JR. Multiplex PCR detection of waterborne intestinal protozoa; Microsporidia, Cyclospora and Cryptosporidium. Korean J Parasitol. 2010. 48:297–301.
14. Ramirez NE, Ward LA, Sreevatsan S. A review of the biology and epidemiology of cryptosporidiosis in humans and animals. Microbes Infect. 2004. 6:773–785.
15. Garcia LS, Bruckner DA, Brewer TC, Shimizu RY. Techniques for the recovery and identification of Cryptosporidium oocysts from stool specimens. J Clin Microbiol. 1983. 18:185–190.
16. Nielsen CK, Ward LA. Enhanced detection of Cryptosporidium parvum in the acid-fast stain. J Vet Diagn Invest. 1999. 11:567–569.
17. Chan R, Chen J, York MK, Setijono N, Kaplan RL, Graham F, Tanowitz HB. Evaluation of a combination rapid immunoassay for detection of Giardia and Cryptosporidium antigens. J Clin Microbiol. 2000. 38:393–394.
18. Sulaiman IM, Xiao L, Lal AA. Evaluation of Cryptosporidium pavum genotyping techniques. Appl Environ Microbiol. 1999. 65:4431–4435.
19. Sharp SE, Suarez CA, Duran Y, Poppiti RJ. Evaluation of the Triage Micro Parasite Panel for detection of Giardia lamblia, Entamoeba histolytica/Entamoeba dispar, and Cryptosporidium parvum in patient stool specimens. J Clin Microbiol. 2001. 39:332–334.
20. Elwin K, Chalmers RM, Roberts R, Guy EC, Casemore DP. Modification of a rapid method for the identification of gene-specific polymorphisms in Cryptosporidium parvum and its application to clinical and epidemiological investigations. Appl Environ Microbiol. 2001. 67:5581–5584.
21. Korea Centers for Disease Control and Prevention. Epidemiologic investigation guideline for food and waterborne diseases 2013 revised ed. 2013. Cheongwon: Division of Epidemic Intelligence Service, Korea Centers for Disease Control and Prevention.
22. Lynch M, Painter J, Woodruff R, Braden C. Centers for Disease Control and Prevention. Surveillance for foodborne-disease outbreaks: United States, 1998-2002. MMWR Surveill Summ. 2006. 55:1–42.
23. Huang DB, White AC. An updated review on Cryptosporidium and Giardia. Gastroenterol Clin North Am. 2006. 35:291–314.
24. Mac Kenzie WR, Hoxie NJ, Proctor ME, Gradus MS, Blair KA, Peterson DE, Kazmierczak JJ, Addiss DG, Fox KR, Rose JB, et al. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. N Engl J Med. 1994. 331:161–167.
25. MacKenzie WR, Schell WL, Blair KA, Addiss DG, Peterson DE, Hoxie NJ, Kazmierczak JJ, Davis JP. Massive outbreak of waterborne cryptosporidium infection in Milwaukee, Wisconsin: recurrence of illness and risk of secondary transmission. Clin Infect Dis. 1995. 21:57–62.
26. Newman RD, Sears CL, Moore SR, Nataro JP, Wuhib T, Agnew DA, Guerrant RL, Lima AA. Longitudinal study of Cryptosporidium infection in children in northeastern Brazil. J infect Dis. 1999. 180:167–175.
27. DuPont HL, Chappell CL, Sterling CR, Okhuysen PC, Rose JB, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med. 1995. 332:855–859.
28. Lee MY, Cho EJ, Lee JH, Han SH, Park YS. A survey of Cryptosporidium oocysts in water supplies during a 10-year period (2000-2009) in Seoul. Korean J Parasitol. 2010. 48:219–224.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download