Abstract
Medical research should be fully transparent. The aims of this study were to determine the prevalence of author-related conflict of interest (COI) policies and evaluate the actual state of COI disclosure in Korean medical journals. To determine the prevalence of author-related COI policies, we examined the 198 medical journals listed in the KoreaMed database. To investigate the actual state of COI disclosures in published papers, we analyzed the publications in a representative medical journal, the Journal of the Korean Medical Science, from the perspective of the relevance of the ethics of COI disclosure. A total of 164 (82.8%) journals required an author's statement of COI as a criterion for publication. Of these 164, most of them focused on financial COI, with 101 (61.6%) presenting the information related to COI disclosures as a separate paragraph with a clear title. We identified 114 articles published by the Journal of the Korean Medical Science over a seven-year period, from January, 2006 to December, 2012. Of these, 65 papers (57%) included an author's statement of COI. We found that the policies of Korean medical journals regarding the disclosure of author COIs are still behind the internationally suggested level.
Conflicts of interest (COI) occur when an author, author's institution, reviewer, or editor has financial or personal relationships that potentially influence his or her actions. These relationships vary from being negligible to having great potential for judgment in research results. Several reports have suggested that data can be significantly favorable to industries that supported the study in question (1, 2). Many studies (3-5) have now revealed a clear association between COIs and research outcomes. For example, Lexchin et al. (4) reviewed 30 studies that they analyzed research sponsored by a pharmaceutical company. They found that studies sponsored by pharmaceutical companies were more likely to have outcomes favoring the sponsor. Also, Bhandari et al. (2) evaluated 332 randomized controlled trials in 13 surgical and 5 medical journals chosen by based on perceived quality and impact factor. They reported that industry-funded trials are more likely to be associated with statistically significant pro-industry findings.
Managing conflicts of interest is critical to protect the rights and welfare of human research participants and to preserve the scientific integrity of the results (6). In an effort to both minimize potential untoward effects and improve public trust, the Institute of Medicine, International Committee of Medical Journal Editors (ICMJE), World Association of Medical Editors (WAME), and Committee on Publication Ethics (COPE) have published ethics guidelines that include specific recommendations for disclosure of COIs as a means to improve transparency. At many journals, policies calling for authors to disclose COIs have evolved as a response to these efforts (7-9).
Recent studies (7, 10) have shown that most medical journals had COI policies for their authors available for public review. Given that there are no reliable data on the prevalence of author's COI policies in Korea, the aim of this study was to determine the prevalence of such policies and evaluate the actual state of COI disclosure in Korean medical journals.
To determine the prevalence of author-related COI policies, we surveyed the KoreaMed database (established by the Korean Association of Medical Journal Editors [KAMJE] and maintained since December 1997) in March 2013. We identified 198 medical journals listed in this database. The guidelines or instructions for authors were examined by accessing either the website or a printed copy of each of the journals. We searched each document for the following phrases related to COIs: "conflict of interest", "financial", "support", "financial relationship", and "funding".
To evaluate the level of COI disclosure in the Korean medical journals, we needed to define our assessment criteria. Two aspects on which we placed major emphasis were the clarity and comprehensiveness of the COI policy of the journal in question.
We defined the clarity and explicitness of COI policies in terms of whether the publication policy dedicated an entire paragraph to guidelines and policies related to the disclosure of COIs, or whether the issue was covered in a single sentence, simple phrase, or just a few words. The extent of the policies related to the disclosure of COIs was assessed by considering whether the journal mentioned both non-financial and financial COIs, financial COIs only, non-financial COIs only, or made no mention of COIs (Table 1). Policies related to funding, payment of honoraria, speaker bureaus, travel expenses, or stock options were included under consideration of financial COIs. The non-financial COI policies, we considered, were related to personal relationships, assistance with writing, academic competition, or political benefits.
Many medical journals revised their ethics guidelines after ICMJE/WAME/COPE recommended COI reporting policies 2008. We also evaluated the level of COI disclosure of the journals before year 2008. Because 'Instruction of authors' on the web-site is the most recent revised guideline, we checked the published book of each of the journals before year 2008.
To investigate the level of compliance and appropriateness of COI disclosures in papers published in Korean medical journals, we investigated recent publications in a representative medical journal, the Journal of the Korean Medical Science (JKMS). This journal is featured in the Science Citation Index Journal and has a purview that spans a wide range of medical fields. We searched medical publications defined by PubMed search criteria such as "dates: published from 2006 to 2012", "type of article: clinical trial", "species: human" and "languages: English". We identified 114 articles listed in this database. Both cover letters and main body associated with the submissions of the published papers were examined to establish whether the authors complied with the journal's request to disclose COIs. For the articles published in JKMS, we determined the frequency with which COIs were disclosed, and evaluated the level of COI disclosure as detailed in Table 1. We also evaluated all of the publications for potential commercial COIs. The potential for a commercial COI was defined as any instance where the publication was supported by industry, or the results implied the effectiveness of a drug, equipment, or software. We checked the study objectives, author's institution, number of authors, publication year, department, and study design in all of the literature examined.
To further assess the current ethical status in the medical literature published in Korea, we analyzed the relevance of ethics in addition to the disclosure of COIs. We conducted ethical analysis suggested by Franklin (11) in terms of: their scientific value, their scientific validity, fairness with which subjects were selected, favorable risk/benefit ratio, independent review, informed consent (IC), and respect for enrolled subjects. We further performed ethical grading based on requirements regarding: IC, approval by an institutional review board (IRB), and transparency of COI disclosure: vague disclosure defined as disclosing about only financial support: transparent disclosure defined as disclosing COI using 'conflict of interest'. Each of the articles was graded on a scale comprising five possible scores (Table 2). If a paper was waived about IC, it was graded on a scale comprising four scores without grade IV.
When all of the authors of the present analysis agreed unanimously with each assessment, the relevance to ethics and ethics grading was accepted. At the end of the study, a specialist of ethics reviewed our assessments.
We identified 198 domestic medical journals in KoreaMed database and evaluated the prevalence of COI policies. Of the included journals, 164 (82.8%) asked that authors provide a statement regarding COIs (Table 3). The guidelines of the remaining 34 (17.2%) journals did not include any comments related to the disclosure of COIs, however, about half of them (19 journals) stated that they followed the Uniform Requirements for Manuscripts Submitted to Biomedical Journals from http://www.icmje.org/.
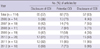
Examination of the clarity and extent of the COI policies of each of the journals in our sample set revealed that 101 (61.6%) of 164 journals presented information related to disclosure of COIs in a dedicated paragraph with a title containing the words "Disclosure of Conflicts of Interest". Twenty-seven (16.5%) of 164 journals presented as explicit word "Conflict of interest" and 36 journals presented as indirect word like as "funding source or financial support". Most of the 164 journals that requested disclosure of COIs focused on financial COIs or not defined about extent of COIs. Approximately one third (28%) requested disclosure of both financial and non-financial relationships, such as personal relationships, self-interest or the provision of writing assistance from authors (Table 3).
We could identify 'instruction for authors' in 105 medical journals published before year 2008. Seventy-one (67.6%) journals asked regarding COIs and 34 (32.4%) journals did not ask disclosure COIs. But, most of them (53 of 71 journals) presented as indirect word and only 14 journals presented in explicit paragraph (Table 4).
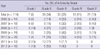
We identified 114 articles (human clinical trial written by English) published by JKMS between January 2006 and December 2012. The guidelines for authors of JKMS clearly require a statement regarding both financial and non-financial COIs in a separate paragraph. We evaluated the prevalence of COIs, the level of COI disclosure, and potential commercial COIs associated with each publication. Of these, 65 papers (57%) contained authors' statements regarding COIs. The remaining 49 papers had no information related to potential COIs (Table 5). Most of the 65 articles scored as disclosing potential COIs were focused on only financial COIs such as "This study was supported by a grant from ooo" or "The authors have no financial interest". Only seven articles disclosed their COIs using the word, COIs. Moreover, just one article informed their COIs in explicit paragraph.
Among the 82 articles (72%), which we regarded as containing potential commercial COI by researchers, only 52 articles contained statements that disclosed the authors' COIs (Table 5). Although, the prevalence of COI disclosure has not improved with time, disclosing COI among the articles which had potential COI showed improvement after year 2010.
We attempted to conduct ethical analysis of the JKMS papers on the basis of seven ethical requirements outlined in the systematic ethical framework shown in Table 2. However, we failed to derive meaningful data according to the systematic ethical analysis suggested by Franklin (11). As an alternative, we instead examined whether the paper had the approval of an IRB and assessed the adequacies of the description of IC, and the disclosure of COIs. Our ethical grading was rated using a five-grade system (Table 2). Of the 114 publications rated in this manner, only seven articles (6%) received the highest grade awarded, as grade I (Table 6).
This is the first report describing the current status of author COI policies and the current state of COI disclosure in Korean medical journals. Our study reveals the prevalence of author-related COI policies and compliance of COI disclosure in Korean medical journals. We found that most (82.8%) of Korean domestic medical journals ask that authors disclose COIs, although half of them state their COI-related policies explicitly in a dedicated paragraph, and about one third of the journals extended the scope of their policies to include non-financial COIs. Given that potential COIs are unavoidable in many clinical situations and types of clinical research, the importance of full transparency in the disclosure of COIs in medical journals and clinical research cannot be underestimated. However, the compliance of COI disclosures in the subject papers was not satisfactory.
COI arises whenever an individual or organization is involved in multiple interests, one of which could possibly corrupt the motivation for an act in the other (12). Medical research should be fully transparent. Otherwise, COIs might seriously influence scientific works and endanger public trust. Guidelines for the disclosure of COIs have been published by ICMJE. It is recommended that when authors submit a manuscript, they are responsible for disclosing all financial and personal relationships on a conflict-of-interest notification page whether potential conflicts do or do not exist. Since then, many medical journals follow the Uniform Requirements. Given the importance of adequate COI disclosure to ensure optimal transparency in medical publications, the majority of medical journals now specifically recommend COI disclosure in their policies (8, 9). Blum et al. (7) have reported the prevalence of author COI policies among medical journals with high impact factors. They found that 89% of these journals required author COI disclosure. Comparison of their findings with those reported by Krimsky and Rothenberg (13) suggests a substantial increase in the prevalence of COI policies over the past decade. Another study by Schneider et al. (14) have reported that 58% of medical journals required author COI disclosure. Of these, 72% journals emphasized the importance of disclosing both non-financial and financial COIs. Our results about the prevalence of COI disclosure seemed to be similar with those reports (82.8%), but considering the conducted study period, the level was not satisfactory. Furthermore, only half of them stated their policy in explicit paragraph and the extent of COI was exclusively focused on financial concerns. The contents of their policy, even though stating in explicit paragraph, were disappointing. Only sixteen medical journals contained definition of COI, boundaries and examples of COI, and how to and whom judge COI. Comparing the status of COI disclosure before year 2008, there were somehow improvements about the prevalence and form of COI policy. However, considering the contents, medical journals have to improve their policy more comprehensive: definition, extent, example, influence, and subject of decision.
Indeed, it is a critical thing that medical journals have policies about COI disclosure for managing conflict of interests. It is more important that readers could easily find disclosure of COIs on published articles. It means that the actual prevalence of COI disclosure, not prevalence of policy about COI, is also important. Although many medical journals have COI policies, the levels of author compliance are not widely investigated. Schneider et al. (14) indicated a very low (15%) prevalence of COI disclosure in published articles, and concluded that readers could not judge the transparency of articles whether COI exist or not. Our research also showed the actual prevalence of COI was low (57%). Almost of them stated their COI about funding sources and only seven articles stated their COI using the word 'conflict of interest'. Considering the COI policy of JKMS, it was unsatisfactory results that almost published articles had disclosed their COI as stated their funding sources. Moreover, many studies would potentially involve commercial COI statements by the authors, disclosures only occur in about 60% of these reports and the frequency of disclosure has not improved over time.
The framework that we deployed for systematic ethical analysis was not a valuable tool for analysis of the 114 JKMS papers describing various clinical trials. This is because Franklin's design feature (11) focused on industry-sponsored placebo-controlled randomized trials, but our design included more various study designs. Instead of Franklin's systemic ethical analysis, our simple ethical grading demonstrated the current level of the relevance to ethics by considering factors such as IC, approval by IRBs, and the transparency of COIs. Generally, IC, approval by IRBs, and COI disclosure has been thought fundamental steps in study design. Disappointingly, only 46 articles rated as grade I and grade II which were satisfied all three components. Besides, the highest grade, transparent COI disclosure, was only 7 articles. However, it was considerable change that no articles rated as grade IV and V after year 2011. We believe that ethical requirements such as these could provide a more valuable tool for IRB members than our grading system for reviewing clinical research protocols, and for peer reviewers and journal editors reviewing manuscripts describing the results of clinical research. We also suggest that an ethical grading system would facilitate a more quick and simple evaluation of the ethical standard of the articles.
Our study has several limitations. We confined out search to the KoreaMed database. Therefore the results are limited by the fact that some journals which were not listed in KoreaMed at the date of search. We reviewed journal policies only by examination of online or printed guidelines for authors. We may have underestimated the prevalence of COI policies. Moreover, given that we confined our search to publications of JKMS for seven years, the data are not representative for all publications of Korea.
In conclusion, we suggest that the policies of Korean medical journals regarding the disclosure of author COIs should be both clearer and more comprehensive. Editors of Korean journals should ask authors mandatorily to disclose potential COIs in a paragraph separate from that describing author's COIs. An IRB of institutions should have its own COI policy to strengthen its review process for scientific integrity and public trust.
Figures and Tables
Table 4
Explicit requirement and extent of disclosure COI before WAME recommended COI reporting policies 2008

References
1. Bell CM, Urbach DR, Ray JG, Bayoumi A, Rosen AB, Greenberg D, Neumann PJ. Bias in published cost effectiveness studies: systematic review. BMJ. 2006. 332:699–703.
2. Bhandari M, Busse JW, Jackowski D, Montori VM, Schünemann H, Sprague S, Mears D, Schemitsch EH, Heels-Ansdell D, Devereaux PJ. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. CMAJ. 2004. 170:477–480.
3. Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA. 2003. 289:454–465.
4. Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ. 2003. 326:1167–1170.
5. Sismondo S. Pharmaceutical company funding and its consequences: a qualitative systematic review. Contemp Clin Trials. 2008. 29:109–113.
6. Morin K, Rakatansky H, Riddick FA Jr, Morse LJ, O'Bannon JM 3rd, Goldrich MS, Ray P, Weiss M, Sade RM, Spillman MA. Managing conflicts of interest in the conduct of clinical trials. JAMA. 2002. 287:78–84.
7. Blum JA, Freeman K, Dart RC, Cooper RJ. Requirements and definitions in conflict of interest policies of medical journals. JAMA. 2009. 302:2230–2234.
8. Flanagin A, Fontanarosa PB, DeAngelis CD. Update on JAMA's conflict of interest policy. JAMA. 2006. 296:220–221.
9. Horton R. A statement by the editors of the Lancet. Lancet. 2004. 363:820–821.
10. Beyer T, Czernin J. Is conflict of interest in our best interest? Eur J Nucl Med Mol Imaging. 2010. 37:1063–1068.
11. Wikipedia. Conflict of interest 2012. accessed on 11 October 2012. Available at
http://en.wikipedia.org/wiki/conflict.
12. Krimsky S, Rothenberg LS. Conflict of interest policies in science and medical journals: editorial practices and author disclosures. Sci Eng Ethics. 2001. 7:205–218.
13. Schneider N, Lingner H, Schwartz FW. Disclosing conflicts of interest in German publications concerning health services research. BMC Health Serv Res. 2007. 7:78.
14. Miller FG, Shorr AF. Ethical assessment of industry-sponsored clinical trials: a case analysis. Chest. 2002. 121:1337–1342.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download