Abstract
The incidence of pulmonary embolism (PE) rises markedly with age, and only a few cases have been reported in younger adults. Thrombophilia has been reported as one of the predisposing factors for PE in younger adults. Here we report an extraordinary case of PE complicated with dysplasminogenemia, a rare genetic disorder resulting in hypercoagulability, in a young male. An 18-yr-old male visited an emergency room in the United States complaining chest discomfort. He was diagnosed as PE with deep vein thrombosis without apparent risk factors. Anticoagulation therapy with warfarin had been initiated and discontinued after 6 months of treatment. After returning to Korea he was tested for thrombophilia which revealed decreased activity of plasminogen and subsequent analysis of PLG gene showed heterozygous Ala620Thr mutation. He was diagnosed with PE complicated with dysplasminogenemia. Life-long anticoagulation therapy was initiated. He is currently under follow-up without clinical events for 2 yr.
Acute pulmonary embolism (PE) is a common clinical problem, often with fatal consequences. The clinical presentation of PE is diverse, ranging from asymptomatic to hemodynamic deterioration. Most cases of PE arise from deep vein thrombosis (DVT) in the calf and about 80% of patients with PE have DVT at the time of presentation (1).
The incidence of PE increases significantly with age. However, it seldom occurs in patients younger than 20 yr (2). Various factors associated with PE such as smoking, immobilization, and malignancy have been identified (3). However, predisposing factors vary somewhat across age groups. Malignancy is rare in younger patients whereas they have a higher incidence of trauma compared to older patients (4).
Plasminogen is a precursor of plasmin, which plays an important role in fibrinolysis by degrading fibrin clots. Few cases of plasminogen deficiency associated with DVT and PE have been reported (5), and the association of plasminogen deficiency with thrombosis is controversial. Here we report an extraordinary case of PE complicated with dysplasminogenemia, a rare genetic disorder resulting in hypercoagulability, in a young male.
An 18-yr-old Korean male high school student studying abroad visited an emergency department on 28 April 2009 in the United States complaining of sudden onset chest pain. The chest pain developed one hour before his visit to the emergency room and was localized to the anterior chest area. His vital signs included a blood pressure of 130/85 mmHg, pulse rate of 96 beats/min, respiratory rate of 20 breaths/min, and body temperature of 36.8℃. His hemoglobin was 16.3 g/dL, WBC count was 6,450/µL (57.3% segmented neutrophils), and platelet count was 175,000/µL. His prothrombin time and activated partial thromboplastin time were 0.98 (INR) (reference range 0.9-1.1) and 32.6 sec (reference range 29.1-41.9 sec), respectively. Electrocardiography showed a normal sinus rhythm without abnormal ST segment change. Chest radiography revealed no abnormality. However, the D-dimer level was elevated to 2.56 µg/mL (reference range < 0.5 µg/mL).
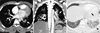
Suspicious of PE, the attending physician ordered a chest computed tomography (CT) scan and duplex ultrasonography of the lower extremity veins. The chest CT scan showed PE in left main pulmonary artery with parenchymal infarction and reactive effusion (Fig. 1). Deep vein thrombus was not visible on duplex ultrasound of the lower extremity veins. As he spent a considerable amount of time studying at a desk, the physician diagnosed him with PE associated with reduced mobility despite the lack of evidence for DVT. Anticoagulation therapy with warfarin was started. After six months of treatment, the anticoagulation treatment was discontinued as he had no risk factors for recurrent PE.
Two months later, the patient returned to Korea and visited the outpatient clinic of Samsung Medical Center on 2 December 2009. Considering the patient's young age, the physician evaluated other risk factors for venous thromboembolism including genetic risk factors for thrombophilia. Protein C and S activity were normal. Anti-phospholipid antibody was absent. However, plasminogen activity was decreased to 59% (reference range 75%-112%). Under suspicion for plasminogen deficiency, a plasminogen gene (PLG) mutation test was performed. The test revealed a PLG mutation of heterozygous Ala620Thr (Fig. 2). The patient's farther and mother were also screened for the PLG mutation and the mother was also positive for PLG mutation of Ala620Thr (Fig. 2). The patient was diagnosed with dysplasminogenemia with PLG mutation Ala620Thr heterozygosity causing venous thromboembolism. Life-long anticoagulation therapy was initiated, and the patient was followed up for 24 months without any clinical events before he returned to the U.S.
Most patients with acute PE have identifiable risk factors at the time of presentation. Common risk factors include immobilization, recent surgery, malignancy, and heart disease. Genetic disorders such as deficiencies in protein C, protein S, and homozygous factor V Leiden are also known to cause thromboembolism (6). However, in some cases, predisposing factors for PE and DVT cannot be identified (3). Identifying predisposing factors for PE is important in deciding the treatment duration and modality and in implementing preventive strategies for recurrence.
Plasminogen deficiency is a rare genetic disorder associated with thrombophilia. In dysplasminogenemia, despite a normal immunoreactive plasminogen level, functional activity is markedly decreased due to abnormalities in the variant PLG molecule (7). Dysplasminogenemia seems to be more prevalent in Asia. Estimated overall incidence of dysplasminogenemia in Korea and Japan is around 1.6%-3.87%, compared to 0.03% in Scotland (8, 9). However, few cases of thrombosis related to dysplasminogenemia in relatively young patients have been reported in Asia (5). In Korea, Song et al. (10) reported that 8.3% of patients with DVT had dysplasminogenemia, suggesting it as a risk factor for thrombosis in the Korean population. However, in heterozygous dysplasminogenemia patients whose plasminogen activity is relatively higher than that of homozygous patients, no increase in risk of thrombosis was revealed (11). In a subsequent population-based study from Japan, isolated dysplasminogenemia, whether heterozygous or homozygous, was not found to be a risk factor for thrombosis (8). Therefore, routine screening for thrombophilia including plasminogen deficiency is not recommended in venous thromboembolism patients (12).
In this case, it was suspected that venous stasis due to a long period of sitting caused DVT and PE. Therefore, anticoagulation was halted after six months of treatment. However, anticoagulation treatment should be maintained throughout life in patients with a thrombophilic tendency. The appropriate diagnosis resulted in modification of the treatment and preventive strategy. Although the benefit of routine screening for dysplasminogenemia in patients with thromboembolism is still debatable, it may be reasonable to check for genetic disorders resulting in hypercoagulability as well as other known risk factors when assessing young patients with PE with or without DVT.
Figures and Tables
References
1. Sandler DA, Martin JF. Autopsy proven pulmonary embolism in hospital patients: are we detecting enough deep vein thrombosis? J R Soc Med. 1989. 82:203–205.
2. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998. 158:585–593.
3. Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012. 379:1835–1846.
4. Green RM, Meyer TJ, Dunn M, Glassroth J. Pulmonary embolism in younger adults. Chest. 1992. 101:1507–1511.
5. Aoki N, Moroi M, Sakata Y, Yoshida N, Matsuda M. Abnormal plasminogen: a hereditary molecular abnormality found in a patient with recurrent thrombosis. J Clin Invest. 1978. 61:1186–1195.
6. Tapson VF. Acute pulmonary embolism. N Engl J Med. 2008. 358:1037–1052.
7. Robbins KC. Classification of abnormal plasminogens: dysplasminogenemias. Semin Thromb Hemost. 1990. 16:217–220.
8. Okamoto A, Sakata T, Mannami T, Baba S, Katayama Y, Matsuo H, Yasaka M, Minematsu K, Tomoike H, Miyata T. Population-based distribution of plasminogen activity and estimated prevalence and relevance to thrombotic diseases of plasminogen deficiency in the Japanese: the Suita Study. J Thromb Haemost. 2003. 1:2397–2403.
9. Schuster V, Hügle B, Tefs K. Plasminogen deficiency. J Thromb Haemost. 2007. 5:2315–2322.
10. Song KS, Lee SM, Choi JR. Detection of an Ala601Thr mutation of plasminogen gene in 3 out of 36 Korean patients with deep vein thrombosis. J Korean Med Sci. 2003. 18:167–170.
11. Shigekiyo T, Kanazuka M, Aihara K, Azuma H, Ohshima Y, Horie H, Nakahira H, Takeichi T, Matsumoto T. No increased risk of thrombosis in heterozygous congenital dysplasminogenemia. Int J Hematol. 2000. 72:247–252.
12. Dalen JE. Should patients with venous thromboembolism be screened for thrombophilia? Am J Med. 2008. 121:458–463.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download