Abstract
Sustained stress can have numerous pathologic effects. There have been several animal models for chronic stress. We tried to identify the changes of pain threshold and hippocampus in a model of chronic stress. Male Sprague-Dawley rats were kept in a cage filled with 23℃ water to a height of 2.2 cm for 7 days. Nociceptive thresholds, expressed in grams, were measured with a Dynamic Plantar Aesthesiometer. Golgi staining was used to identify hippocampal changes. To demonstrate how long allodynia was lasting, behavioral test was repeated daily on another experiment. Compared to control group, chronic stress group showed bilateral mechanical hyper-responsiveness on days 5 (P = 0.047) and 7 (P = 0.032). In general, dendrite atrophic changes within hippocampus of chronic stress model were much more prominent in comparison with control. Compared to control, decreased spine number (P < 0.001) and spine length (P < 0.001) on Golgi staining were seen in the hippocampus of animals with chronic stress. Bilateral mechanical hyperresponsiveness was recovered on day 19 in animals with chronic stress. Chronic stress may bring about central sensitization and hippocampal changes in rats.
Chronic stress is an ongoing or repeated exposure to one or more types of stress stimuli over a period ranging from days to months (1). Sustained stress can have numerous pathologic effects. For example, stress of a few days endangers hippocampal neurons and sustained stress can damage the human hippocampus (2). It is also reported that chronic restraint causes retraction of apical dendrites and reduces spine density in pyramidal cells in the hippocampus and prefrontal cortex (3, 4). Although there have been several animal models for chronic stress, Tanaka et al. (5) recently established an unique model in rat. In that model, rats were kept in a cage filled with water to a height of 2.2 cm for 5 days. Under this condition, they were exposed to continuous stress and could not maintain a resting position for long periods.
There are several reports of the connection between hippocampus and allodynia (6, 7). Hippocampal transient receptor potential vanilloid 1 (TRPV1) can modulate central pain processing (6). The formalin-induced increase in 5-hydroxyindoleacetic acid (5-HIAA) in the hippocampus was significantly blunted in the olfactory bulbectomized (OB) versus sham-operated rats. Correlation analysis determined a significant inverse correlation between formalin-evoked nociceptive behavior and 5-HIAA concentration in the hippocampus (7).
Since the hippocampus inhibits brain centers associated with the stress response (8), we hypothesized that chronic stress may cause allodynia due to hippocampal damage. To prove this hypothesis we examined the morphology of hippocampal neurons in an animal model of chronic stress.
Male Sprague-Dawley rats (7 weeks old) were obtained from Orient Bio Inc. (Seongnam, Korea). Rats were housed in a temperature (20℃-23℃) on a 12-2-hr light-dark cycle. Food and water were available ad libitum. The experimental animals were kept in a cage filled with water (23℃ ± 1℃) to a height of 2.2 cm for 7 days (experiment 1, n = 5; experiment 2, n = 10). A nontreated control group was also included (experiment 1, n = 5; experiment 2, n = 10). The animal studies were performed after receiving approval of the institutional animal care and use committee (IACUC) in Dongguk University (IACUC approval No. 2009-036) and was carried out in accordance with the guidelines of the International Association for the Study of Pain policies on the use of laboratory animals.
Animals were brought to the testing room an hour before behavioral testing to acclimatize them to the testing environment. Behavioral tests were usually done between 10 am and 3 pm. Rats were separated into two study subgroups according to water immersion (group 1, with water immersion; group 2, without water immersion). Both side paw withdrawal responses were measured in response to mechanical stimuli at days 0 (baseline), 5, and 7 with a Dynamic Plantar Aesthesiometer (Ugo Basile, Comerio VA, Italy) (Experiment I, n = 5; group 1 and 2, respectively). To demonstrate how long allodynia was lasting, behavioral test was repeated daily on another experiment (Experiment II, n = 10; group 1 and 2, respectively). The aesthesiometer consists of a movable force-actuator below a platform on which the rat is resting. The actuator is placed beneath the hindpaw and the actuator confers a defined force on a von Frey-type filament. The filament exerts increasing force to the plantar surface of the hindpaw, starting below the threshold of detection and increasing until the animal removes its paw. When the paw is withdrawn, the device registers and displays the force (in grams) at which paw withdrawal occurred. On a given test day, the same procedure is repeated 3 times and the average force to produce a withdrawal computed. A maximal cut-off value of 50 g was used to prevent tissue damage.
After behavioral testing was completed on day 7, animals were anesthetized with sodium pentobarbital (3.0-3.5 mL/kg, i.p.), and perfused with saline solution followed by 4% paraformaldehyde in phosphate buffer. After that, fresh brains were removed, hippocampal formations were dissected and fixed in 10% formalin for 24 hr. The tissues were transferred into 3% potassium bichromate for 5 days with solution changes each day, followed by 2% silver nitrate for 3 days at room temperature in the dark. Solutions were changed several times until brown participates did not appear. After rinsing in distilled water, each hippocampus was cut into three equal parts. The middle parts of the hippocampi were frozen in Tissue-Tek® O.C.T™ Compound (Sakura Finetek USA, Inc., Torrance, CA, USA), and coronal sections of 20 µm thickness were obtained using a cryostat (Tissue-Tek® Cryo3® Microtome/Cryostat, Sakura Finetek USA Inc.). Sections were collected on clean, 2% gelatin-coated glass microscope slides, and mounted with Permount (Thermo Fisher Scientific Inc., Waltham, MA, USA) diluted in xylene (1:3).
Spines of pyramidal neurons in the hippocampal CA1 area were evaluated. Pyramidal neurons were readily identified by their characteristic triangular soma shape and relative position in the hippocampal formation. The number of spines in 20 µm segments (n = 30) of typical CA1 pyramidal neurons (n = 20) from both hemisphere were counted by a student blind to the experimental procedures. The spine length was defined the distance from the border of dendritic shaft to the end of spine head. Spine density and length were analyzed using a two-way ANOVA, followed by Mann-Whitney U test. P values less than 0.05 and 0.01 were considered being significant and very significant.
Compared to control group, chronic stress group showed bilateral mechanical hyper-responsiveness on day 5 (P = 0.047) and day 7 (P = 0.032) (Fig. 1). Data from left and right hindpaws collapsed together for clarity of presentation. Mean and SD for the force applied were 33.4 ± 9.8 g and 27.6 ± 5.0 g on day 0, 34.7 ± 10.0 g and 21.6 ± 5.2 g on day 5, 29.0 ± 14.7 g and 15.3 ± 4.4 g on day 7 (control and chronic stress group, respectively).
In general, dendrite atrophic changes within the hippocampus of chronic stress model were much more prominent in comparison with control on Golgi staining. Compared to control, decreased spine number (P < 0.001) and spine length (P < 0.001) on Golgi staining were seen in the hippocampus of animals with chronic stress (Fig. 2).
Comparison of spine number per 20 µm between control and stress group in the hippocampus showed 12.5 and 8.7, respectively (Fig. 2C). Comparison of average spine length between control and stress group in the hippocampus showed 1.31 and 0.78 µm, respectively (Fig. 2D).
To demonstrate how long allodynia was lasting, behavioral test was repeated daily on another experiment until about 6 weeks (Experiment II). Fig. 3 shows the median mechanical threshold from stress and control animals according to observation day. Bilateral mechanical hyper-responsiveness was recovered on day 19. Data from left and right hindpaws collapsed together for clarity of presentation (Fig. 3).
This study demonstrated the first evidence of changes in pain threshold and the hippocampus in an animal model of chronic stress. This study examined stress induced changes in sensitivity to mechanical stimuli and in hippocampal cell morphology in rats. Prolonged exposure of the lower body to slightly cold water impeded rest and sleep in the animals. Mechanical paw withdrawal thresholds were lowered in the stressed animals compared to a group of control animals. Changes in dendritic spines were observed in the hippocampal cells compared to controls. The changes in hippocampal cell morphology are of potential significance because the hippocampus is a part of the limbic system circuitry and is connected to the hypothalamic-pituitary-adrenal (HPA) axis and these brain components are closely implicated in mediating diseases such as fibromyalgia (FM) and chronic stress (9, 10). Since repeated stress produces neuronal remodeling in the amygdala, prefrontal cortex, and hippocampus (11), these hippocampal changes are expected.
Tanaka et al. (5) established an animal model of stress and examined the mechanisms underlying central fatigue. In the model, animals subjected to an environment in which it was impossible to rest mentally and physically would progressively become fatigued. Under such a condition, as they would try to avoid water, they could not assume a resting position or sleep soundly. In practice, at best they only took a short sleep with their shoulders against the side of the cage. The stressed animals showed a progressively shorter swimming time. It was hard for the rats placed in water for 7 days to recover. The rats kept in water for 5 days showed the shortest swimming time and all of these animals could show recovery. Therefore, we chose day 5 and day 7 for pain threshold test and hippocampal changes on the first experiment. For clarification of daily allodynia change, we had another study from baseline to at least 6 weeks in this animal model of chronic stress.
The hippocampus is an integral component of the limbic system, and as such may contribute to the negative affect and avoidance motivation experienced during pain. A substantial body of evidence indicates that the hippocampus processes pain-related information, and some hippocampal neurons respond exclusively following noxious stimulation (12). The hippocampus participates in nociception, a function positively correlated with the activity of hippocampal N-methyl-D-aspartate (NMDA). Several stress-related hormones are known to enhance the activity of hippocampal NMDA receptors, increasing excitatory neurotransmission within the hippocampus (13). Blocking NMDA receptors in the hippocampal formation reduces nociceptive behaviors; this in turn supports the hypothesis that the hippocampal formation is involved in pain-related neural processing and expression of pain-related behaviors (12). In the most recent study, hippocampus was dysfunctional in patients with FM, as shown by lower N-acetylaspartate (NAA) levels compared to controls, representing neuronal or axonal metabolic dysfunction. As the hippocampus plays crucial roles in maintenance of cognitive functions, sleep regulation, and pain perception, they suggest that metabolic dysfunction of hippocampus may be implicated in the appearance of these symptoms associated with FM (9). In the other recent study, Wood et al. (14) have demonstrated an abnormality in hippocampal brain metabolites in premenopausal female FM patients. A significant negative correlation between patient subjective experience of symptoms and a reduced NAA/creatine ratio suggests a role for hippocampal pathology in FM. These findings demonstrate the involvement of hippocampus is critical in FM pathogenesis. Therefore, animal research such as this model is needed to further clarify the role of hippocampal pathology in FM because the specimen is easier to get in rodents.
What is the relationship between spine number and morphology and pressure pain thresholds? It is unclear if the changes in spine number and morphology are related to pain symptoms or other aspects of this model such as prolonged stress. In the experimental animal data, rapid eye movement (REM) sleep deprivation was observed to enhance pronociception in almost all studies (15). The exclusive focus on REM sleep deprivation (easier to accomplish in rodents) does not resolve issues regarding the effects of non-REM sleep deprivation. Thus, it might be the case that the observed effects are specific to REM sleep deprivation and are not due to a general and unspecific disruption of sleep (15). However, this chronic stress model at best only take a short sleep with their shoulders against the side of the cage, and so is different from previous sleep deprivation model.
The reported increases in pressure sensitivity were long lived and these effects were lasting up to 2 weeks after allodynia developed. The stress response has classically been characterized by two temporal 'waves' of stress mediator actions. The first includes rapid actions of noradrenaline, serotonin, dopamine and corticotropin-releasing hormone. These rapid actions of stress mediators promote vigilance, alertness, appraisal of the situation and the choice of an optimal strategy to face the challenge. Because local increases in stress mediator levels are shortlived, and because their actions typically quickly subside, this first wave of events is not optimal for provoking the sustained, adaptive components of a stress response. This is instead accomplished through alterations of gene expression and cell function, classically considered the second wave and attributed to corticosteroids acting through glucocorticoid receptors within hours-days-months time frame (10). The recovery of allodynia after 2 weeks in our study may also be attributed to a stress response of corticosteroids. Further research is required to show the association between allodynia recovery and corticosteroids in this model.
The limitation of this study was that there was no assessment of reversibility considering the impressive morphological results. A similar model "forced swim model of stress-induced hyperalgesia" is known for demonstrating widespread mechanical and chemical hyperalgesia and has also shown changes in the serotonergic system and in the HPA system (16).
In conclusion, chronic stress may bring about central sensitization and hippocampal changes in rats. A number of chronic pain states including FM, irritable bowel syndrome, tension headache, etc. are thought to be triggered by stress and are accompanied by some of these same changes.
Figures and Tables
Fig. 1
Response to mechanical stimulation. Stress model showed bilateral mechanical hyper-responsiveness on day 5 and 7 compared to control. (Experiment I: n=5, group 1 and 2, respectively) The error bars indicate SD. Data from left and right hindpaws collapsed together for clarity of presentation. *P < 0.05.

Fig. 2
Spine number and length changes on Golgi staining within the hippocampus of rats. (A) No significant changes of spine number and length within the hippocampus of control rats (×100). The rectangle of left is enlarged on the right. (B) Significantly decreased spine number and length within hippocampus of stress model (×100). The rectangle of left is enlarged on the right. (C) Comparison of spine number per 20 µm between control (12.5) and stress (8.7) group in the hippocampus. (D) Comparison of spine length between control (1.31 µm) and stress (0.78 µm) group in the hippocampus (Experiment I: n = 5, group 1 and 2, respectively). Bars show the mean and SD. *P < 0.001 vs control.
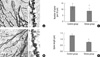
Fig. 3
Line graph representing the median mechanical threshold from stress and control animals according to observation day. Bilateral mechanical hyper-responsiveness was recovered on day 19 (Experiment II: n = 10, group 1 and 2, respectively). Data from left and right hindpaws collapsed together for clarity of presentation. *P < 0.05, †P < 0.01, ‡P < 0.001.

Notes
References
1. Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009; 10:397–409.
2. Sapolsky RM. Why stress is bad for your brain. Science. 1996; 273:749–750.
3. Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995; 69:89–98.
4. Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008; 507:1141–1150.
5. Tanaka M, Nakamura F, Mizokawa S, Matsumura A, Nozaki S, Watanabe Y. Establishment and assessment of a rat model of fatigue. Neurosci Lett. 2003; 352:159–162.
6. Wu YW, Bi YP, Kou XX, Xu W, Ma LQ, Wang KW, Gan YH, Ma XC. 17-Beta-estradiol enhanced allodynia of inflammatory temporomandibular joint through upregulation of hippocampal TRPV1 in ovariectomized rats. J Neurosci. 2010; 30:8710–8719.
7. Burke NN, Hayes E, Calpin P, Kerr DM, Moriarty O, Finn DP, Roche M. Enhanced nociceptive responding in two rat models of depression is associated with alterations in monoamine levels in discrete brain regions. Neuroscience. 2010; 171:1300–1313.
8. Wood PB. Fibromyalgia syndrome: a central role for the hippocampus: a theoretical construct. J Musculoskelet Pain. 2004; 12:19–26.
9. Emad Y, Ragab Y, Zeinhom F, El-Khouly G, Abou-Zeid A, Rasker JJ. Hippocampus dysfunction may explain symptoms of fibromyalgia syndrome: a study with single-voxel magnetic resonance spectroscopy. J Rheumatol. 2008; 35:1371–1377.
10. Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009; 10:459–466.
11. McEwen BS, Chattarji S. Behavioral neurochemistry and neuroendocrinology. In : Lajtha A, editor. Handbook of Neurochemistry and Molecular Neurobiology. 3rd ed. New York: Springer;2007. p. 571–594.
12. McKenna JE, Melzack R. Blocking NMDA receptors in the hippocampal dentate gyrus with AP5 produces analgesia in the formalin pain test. Exp Neurol. 2001; 172:92–99.
13. Schuff N, Amend DL, Knowlton R, Norman D, Fein G, Weiner MW. Age-related metabolite changes and volume loss in the hippocampus by magnetic resonance spectroscopy and imaging. Neurobiol Aging. 1999; 20:279–285.
14. Wood PB, Ledbetter CR, Glabus MF, Broadwell LK, Patterson JC 2nd. Hippocampal metabolite abnormalities in fibromyalgia: correlation with clinical features. J Pain. 2009; 10:47–52.
15. Kundermann B, Lautenbacher S. Effects of impaired sleep quality and sleep deprivation on diurnal pain perception. In : Lavigne GS, Sessle BJ, Choinière M, Soja PJ, editors. Sleep and Pain. Seattle: IASP Press;2007. p. 137–152.
16. Quintero L, Moreno M, Avila C, Arcaya J, Maixner W, Suarez-Roca H. Long-lasting delayed hyperalgesia after subchronic swim stress. Pharmacol Biochem Behav. 2000; 67:449–458.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download