Abstract
This study was done to evaluate whether injections of resveratrol, a natural compound found in the skin of grapes, had anabolic effects on degenerated intervertebral discs in a rabbit model. Two non-continuous lumbar discs were punctured in rabbits to induce disc degeneration. Four weeks and 6 weeks after puncture, the rabbits were treated by injections with dimethylsulfoxide (DMSO) or resveratrol. At 4, 8, and 16 weeks after initial injection, rabbits were sacrificed and the spine was extracted for magnetic resonance image (MRI), mRNA expression, and histological staining. Resveratrol treatment resulted in stronger signal intensity in T2-weighted images. MRI grade showed significantly lower in the resveratrol group than the DMSO group (P = 0.039). In the resveratrol group, aggrecan gene expression was significantly increased than that in the DMSO group at 16 weeks after injection (P = 0.027). MMP-13 mRNA levels in the resveratrol group were significantly decreased than those in the DMSO group at 8 and 16 weeks (P = 0.006 and P = 0.048, respectively). In hematoxylin and eosin stain, resveratrol-treated discs showed the features of regeneration. Histologic grade revealed improvement in resveratrol-treated discs, compared with DMSO-treated discs (P = 0.024). These anabolic effects on degenerated discs indicate that resveratrol is a promising candidate for treatment of degenerative disc disease.
Low back pain is a common and well-known incapacitating disorder. Despite the multifactorial etiology of back pain, intervertebral disc (IVD) degeneration is one of the major factors that causes this disabling condition (1, 2). Treatments are currently limited to improving symptoms and do not aim to impede early pathophysiological processes involved in degeneration. Current treatments include medications, steroid injection, physical therapy, and surgery. Surgical treatment can be in the form of fusion or other procedures that are destructive to the IVD. The long-term effects of newer treatment methods, such as intradiscal electrothermal therapy and artificial discs, still have to be evaluated. IVDs could also potentially be repaired or regenerated using peptide or growth factor injections, gene delivery, cell therapy, or various tissue engineering techniques (3-8).
Resveratrol (trans-3,4´,5-trihydroxystilbene) is a polyphenolic phytoalexin present in the skins of grapes and red wines; it has been shown to be a potent and specific inhibitor of nuclear factor kappa B (NF-κB) activation (9-11). NF-κB is a key transcription factor involved in the activation of the interleukin-1beta (IL-1beta) and tumor necrosis factor-alpha (TNF-alpha) pathways, which play a pivotal role in degenerated and herniated IVDs (12-14). Resveratrol has been reported to antagonize catabolic factor-mediated up-regulation of matrix-degrading enzymes and to promote proteoglycan synthesis and accumulation in bovine disc cells (15), as well as to reduce levels of proinflammatory cytokines and catabolic enzymes in human IVD cells (16).
The purpose of the present study was to determine whether resveratrol had anabolic and/or anti-catabolic effects on disc degeneration following intradiscal injection in an experimental rabbit model.
All experiments in the present study were performed with approval from the institutional animal care and use committee (IACUC) of the Samsung Biomedical Research Institute, Seoul, Korea (IACUC approval No. 72). Twenty-four New Zealand white rabbits (4-5 months old and weighing approximately 3-3.5 kg each) were subjected to annular puncture of lumbar discs. The disc degeneration model was established using an 18-gauge angiography needle by percutaneous annular puncture technique (Fig. 1), modified from previous open surgical techniques (17, 18).
Each rabbit was anesthetized by intramuscular injection of xylazine (5 mg/kg) and ketamine (35 mg/kg), and hair was shaved from the mid back and right flank. After anesthesia, the rabbits were placed in a lateral oblique prone position. An alcohol sponge was used to sterilize the surgical field. Initially, the L5-L6 disc was identified by manual palpation of the interspinous space from the mid back and iliac crest using fluoroscopy (VPX-200; Toshiba, Irvine, CA, USA). After confirmation of the exact level, an 18G angiography needle was inserted, 3-4 cm ventral from the midline, into the disc space under fluoroscopic control. Correct needle positioning in the center of the disc space was checked from both anteroposterior and lateral views. In each rabbit, L2-L3 and L4-L5 IVDs were punctured. The L3-L4 level was left non-punctured and used as an internal normal control. Special care was taken to minimize touching the periosteal tissues of the vertebrae because this could cause hypertrophy of the soft tissues and bony structures around the discs. None of the rabbits showed neurological symptoms after surgery, and recovery was uneventful.
Four weeks after the initial puncture, degeneration of the punctured discs was identified by measuring decreases in disc height with lateral X-rays. Rabbits were divided into two groups according to the treatment they received (control vs treatment groups). The stable and pharmacologically active trans-isomer form of resveratrol (Sigma, Saint Louis, MO, USA) was purchased, and prepared according to the manufacturer's protocol. Resveratrol was dissolved in dimethylsulfoxide (DMSO) and used at a concentration of 100 µM.
The left flank was injected using the same anesthesia protocol. Rabbits in the treatment group received injections of 15 µL of 100 µM resveratrol in DMSO into the center of the nucleus pulposus using a 26-gauge needle. At the same time, the control group was injected with 15 µL of DMSO. Injections were repeated two weeks later in each group. Then, four rabbits in each group were euthanized by intramuscular injection of ketamine (25.0 mg/kg) followed by intravenous injection with sodium pentobarbital (1.2 g/kg) at 4, 8, and 16 weeks after the initial injection. The entire spinal column from each animal was extracted for magnetic resonance image (MRI) analysis. Discs from the spine column were subjected to biochemical and histological analyses.
MRI scans were performed on ex vivo spinal columns harvested from rabbits at three time points: 4 weeks, 8 weeks, and 16 weeks after the initial injection. MRI was performed using a 1.5-T machine (Intera Release 11; Philips Medical System, Best, The Netherlands) with a quadrature extremity coil (knee coil). T2-weighted sections in the sagittal plane were obtained using the following settings: fast spin echo sequence with time to repetition (TR) of 3,000 msec and time to echo (TE) of 100 msec; 256 × 189 matrix; 140 × 140 field of view and 6 number of signal averaging. The section thickness was 2 mm with a 0-mm gap. The MRIs were sent to a picture archiving and communication system server and analyzed using the PiViewSTAR program (INFINITT Healthcare, Seoul, Korea). The images were quantified using a modified Thompson grade based on changes in the degree and area of signal intensity from grade 1 to 4 (1, normal; 2, minimal decrease in signal intensity but obvious narrowing of high signal area; 3, moderate decrease in signal intensity; and 4, severe decrease in signal intensity). Two blinded observers checked the grading for each image, and the average grade was used as the final grade for each disc. MRI data from all three time points were pooled and analyzed according to treatment group.
To analyze changes in gene expression in the disc tissue, RNA from three discs (L2-L3, L3-L4, and L4-L5) from each spinal column was extracted after MRI data acquisition. The L3-L4 disc was used as a non-injected normal control. For each disc, the nucleus pulposus (NP) was carefully removed from the annulus fibrosus (AF) and stored separately. Tissues were immediately placed in liquid nitrogen and then frozen at -80℃ before use in real time reverse transcriptase-polymerase chain reaction (RT-PCR) analysis.
The author selected an extracellular matrix (ECM) gene, aggrecan, and a catabolic enzyme gene, matrix metalloprotease-13 (MMP-13), as marker genes. RNA was extracted from homogenized NP samples using Trizol (Invitrogen, Carlsbad, CA, USA) and cDNA was generated using the Moloney murine leukemia virus (MMLV) reverse transcriptase (RT) (Invitrogen). Gene expression was analyzed by real time RT-PCR using an ABI PRISM 7900 instrument (Applied Biosystems, Foster City, CA, USA) and evaluated in Microsoft Excel.
For each sample, gene expression levels were calibrated using a constitutively expressed housekeeping gene, glyceraldehyde phosphate dehydrogenase (GAPDH). To quantitate gene expression, the comparative Ct method was used. The difference between the mean Ct value of the gene of interest and the housekeeping gene was denoted (ΔCt), and the difference between ΔCt of the treated sample and ΔCt of the normal sample was labeled ΔΔCt. Log2 (ΔΔCt) gave the relative difference in gene expression between the treated disc and the normal control disc in each animal. Rabbit-specific gene primers were designed according to the published sequences available in GenBank. The sequences were as follows: aggrecan (5´ GCTACGGAGACAAGGATGAGTTC 3´ and 5´ CGTAAAAGACCTCACCCTCCAT 3´), MMP-13 (5´ TGCCCCTCCTCAACAGTAAC 3´ and 5´ GAGCCCGCTGCATTCTTCTT 3´), GAPDH (5´ AAGGCCATCACCATCTTCCA 3´ and 5´ GGATGCGTTGCTGACAATCT 3´).
The injected discs (L2-L3 and L4-L5) and the normal control disc (L3-L4) of rabbits were cut together with the adjacent vertebral body using an electrical saw. Tissues were fixed in 10% neutral buffered formalin for 48 hr, decalcified in decalcification solution (National Diagnostics, Atlanta, GA, USA) for 3 days, and processed for paraffin sectioning. Blocks of discs were embedded in paraffin and cut into mid-sagittal sections (4 µm in thickness) using a microtome. Sections were stained with hematoxylin and eosin (H&E) to determine the shape and number of cellular constituents as well as the amount of ECM, and then analyzed quantitatively under a light microscope (Nikon Eclipse E800; Nikon, Melville, NY, USA) at magnifications ranging from 40 × to 100 × .
To quantify histological grade, The author used a grading scale previously established in rabbits (17). A blinded observer evaluated the midsagittal sections of each disc using this grading scale. The scale was designed to assess the shape of the AF, the border between the AF and the NP, the cellularity of the NP, and the matrix of the NP. Grades range from 4 to 12. A normal grade was assigned 1 point for each of four categories through the midsagittal sections, for a total of 4 points. Because 3 points was the maximal value for each parameter, a total of 12 points was the highest score for degeneration. The overall discs gathered from the three time points were analyzed. The treated discs were initially compared with the internal normal control disc within the same animal to evaluate changes due to age, and graded numerically in both control and treament groups.
Statistically significant differences in MRI grade and total histological grading score between the DMSO group and resveratrol group were assessed using the non-parametric Mann-Whitney U test. Quantitative real time RT-PCR results were analyzed with t test. Data were analyzed using PASW Statistics 18.0 (IBM, Armonk, NY, USA). Data are presented as mean ± standard deviation. P values less than 0.05 were considered statistically significant.
MRIs obtained from spinal columns 4 weeks after the initial injection had stronger T2-weighted signal intensities in the resveratrol group than in the DMSO group. Similar results were also observed at 8 weeks and 16 weeks. Representative MR images of the two punctured discs revealed restoration of the signal intensities and areas of the discs in resveratrol-treated rabbits compared to DMSO-treated rabbits (Fig. 2A, B). Modified Thompson MRI grade scores, which indicate the degree of disc degeneration, were significantly lower in the resveratrol-treated rabbits than the DMSO-treated rabbits (P = 0.039) (Fig. 2C).
The gene expression results of the control and test groups were analyzed for each time period (4 weeks, 8 weeks, and 16 weeks after initial injection) (Fig. 3). All results are reported as relative percentages compared with the initial, non-punctured levels of mRNA for each respective gene. The overall gene expression of a representative ECM component, aggrecan, in the DMSO group was well below normal levels for the entire study period. Aggrecan mRNA in DMSO-treated discs was 65.4% of that of non-injected normal discs at 16 weeks. Gene expression increased to 123.0% in the resveratrol-treated disc group. In the resveratrol group, aggrecan gene expression was significantly higher than that in the DMSO group at 16 weeks after injection (P = 0.027). MMP-13 m RNA levels in DMSO-treated discs gradually increased over time. In the resveratrol group, MMP-13 mRNA levels were maintained lower level than those in DMSO group. Significant differences were observed at 8 and 16 weeks (P = 0.006 and P = 0.048, respectively).
Resveratrol injection also affected the histologic appearance of discs. H&E staining of non-punctured normal discs revealed clear demarcations between the NP and the AF, and abundant ECM (Fig. 4A, a). NP cells consisted mainly of large, vacuolated notochordal cells and chondrocyte-like cells. Disc tissues of resveratrol-treated discs exhibited regenerative characteristics similar to those of the normal disc. High-power field observation revealed grape-like clusters of large, round chondrocytic cells in the NP of the resveratrol-treated discs (Fig. 4c). In contrast, DMSO-treated discs lost integrity of the NP margin and showed degenerated cell features such as fibroblast-like cells and severe fibrosis of extracellular components (Fig. 4B, b). Comparison of the overall grades between treatment groups revealed that the degeneration grades of the punctured discs in the resveratrol group were significantly lower than those in the DMSO group (P = 0.024) (Fig. 4D).
The pathogenesis of IVD degeneration is not yet fully understood. A number of cytokines have been implicated in the pathogenesis of IVD degeneration. IL-1beta and TNF-alpha are the predominant proinflammatory cytokines related to disc degeneration (12-14). It follows that regulation of these proinflammatory cytokine signaling pathways may slow or prevent disc degeneration. NF-κB is an important transcription factor involved in activation of the IL-1beta and TNF-alpha signaling pathways (10, 19, 20). Activation of NF-κB has been shown to play a key role in inflammation and hyperplasia in rheumatoid arthritis (21, 22). NF-κB also mediates the expression of proinflammatory cytokines in osteoarthritis (20, 23).
Resveratrol has anti-inflammatory, anti-oxidant, anti-tumoral, and immuno-modulatory effects (9-11, 24). Its potential use for the treatment of osteoarthritis has been investigated in in vitro studies (19, 20). Resveratrol reduced both IL-1beta-induced NF-κB activation and NF-κB dependent gene expression, thereby attenuating apoptosis, inflammation, and matrix degradation in chondrocytes. An animal study demonstrated that resveratrol had an anabolic effect on cartilage tissue. These authors reported a significant reduction in cartilage destruction and loss of matrix proteoglycans in the resveratrol-treated group compared to the control group (25).
The anti-inflammatory and anti-catabolic effects of resveratrol on disc degeneration have been well documented. Resveratrol can reduce transcript and protein expression of major proinflammatory cytokines (IL-6 and IL-8) and matrix catabolic enzymes (MMP-1, MMP-3, and MMP-13); these cytokines and catabolic enzymes have previously been shown to induce pain and disc degeneration (16). An in vitro study using a three-dimensional alginate culture system that mimicked in vivo conditions demonstrated that resveratrol increased proteoglycan synthesis in a dose-dependent manner and rescued IL-1beta-induced proteoglycan loss in bovine IVD cells (15). Resveratrol has also been reported to have anti-inflammatory and pain behavior effects in vivo in a rabbit model of radiculopathy (16). Pain provocation by the application of NP onto the dorsal root ganglion was effectively controlled after local injection of resveratrol.
The author performed this in vivo study to determine the effect of resveratrol treatment in a degenerated disc rabbit model. The model using annular puncture provides reproducible mild degeneration, and is useful for quantitative and semiquantitative analyses of various parameters (17, 18). By radiological, biochemical, and histological methods, it can be used to verify treatment efficacy. The author modified this technique to make it minimally invasive using percutaneous puncture under fluoroscopic guidance. This modification enabled repeated injection of resveratrol without open surgery, which can stress animals.
The author demonstrated that intradiscal injection of resveratrol had anabolic and anti-catabolic effects on IVD degeneration as assessed by MRI, real time RT-PCR, and histological analyses. MRI allowed intuitive visual quantification using four numeric grades. MRI with a T2-weighted protocol demonstrated that two time injections of resveratrol increased the signal intensity and area of the degenerated disc compared to control discs. These results suggest that the proteoglycan content in the disc was increased by resveratrol treatment because the electronegative charges on the glycosaminoglycans of proteoglycan are known to increase osmotic and hydrostatic pressure, resulting in swelling of the disc (26). Previous in vitro findings (15) of the anabolic effect of resveratrol on proteoglycan are consistent with these MRI findings.
The expressions of two representative genes were analyzed according to time interval after injection. mRNA levels of aggrecan, a characteristic product of chondrocyte-like NP cells, have been shown to decrease at 3 weeks and thereafter without spontaneous recovery in degenerated discs in a rabbit model (27). The author obtained similar results in this study when examined degenerated, DMSO-treated discs. Aggrecan mRNA levels in the degenerated discs were lower than in normal control discs throughout the experimental period. In contrast, resveratrol-treated discs showed increased expression of aggrecan mRNA compared to that in DMSO-treated discs. MMP-13, also known as collagenase-3, is a major catabolic enzyme that is expressed at high levels in degenerative arthritic diseases. In another degenerated disc rabbit model (28, 29), MMP-13 mRNA levels were increased at various time points after induction of degeneration. The author found that MMP-13 transcript levels were increased in the DMSO group, and that this increase was significantly suppressed in the resveratrol group. These results suggest that resveratrol injection increased the ECM component and decreased the catabolic enzyme in IVDs, thereby promoting renewal of degenerated discs.
The histological findings showed confirmatory characteristics of disc renewal in this study. In previous studies of disc degeneration using a rabbit model, annular puncture resulted in the replacement of large notochordal cells with small chondrocytic or fibroblast-like cells, which are indicators of disc degeneration (17, 18). In the present study, the author observed fibroblast-like cells in the degenerated, DMSO-treated discs. In contrast, improvement of the integrity of the NP and AF was observed in resveratrol-treated discs, as well as increased extracellular components and cellularity in the central nucleus. NP cells had typical chondrocytic features, suggesting reversal or blockage of the degenerative process. These histologic features of rejuvenated degenerated disc cells have been also described previously (3, 6, 7).
In this study, degenerated discs were treated with two repeated injections of a single dose of resveratrol or vehicle (DMSO). Pharmacodynamic in vivo studies with various doses are required to confirm the maximum effective dose; furthermore, the long-term efficacy of resveratrol needs to be verified in studies with an extended follow-up period after the injection.
The present study demonstrated that resveratrol had anabolic effects on degenerated disc by performing radiological, biochemical, and histologic studies. Resveratrol increased aggrecan and decreased in MMP-13 in the transcriptional level. Subsequently it enhances the synthesis of matrix proteoglycan and diminishes the degradation of matrix collagen in disc tissues, and could show the anabolic effect on degenerated discs. These results indicate that resveratrol has significant potential as a disease-modifying drug for treatment of degenerative disc disease.
Figures and Tables
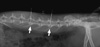 | Fig. 1Percutaneous needle puncture into the rabbit discs under a fluoroscope. Generally, a rabbit has seven lumbar vertebrae. After localization of the exact levels of L2-L3 and L4-L5 (arrows) using the fluoroscope, 18 G angiography needles (radio opaque materials in the figure) are inserted into the discs under a real time monitoring. |
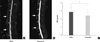 | Fig. 2Ex vivo analyses of magnetic resonance image (MRI) between the dimethylsulfoxide (DMSO) group and the resveratrol group. Representative T2-weighted sagittal image of lumbar spine demonstrated that resveratrol-treated discs (B) showed higher signal intensity than DMSO-treated discs (A) (arrows; treated discs of L2-L3 and L4-L5). Analysis of MRI data pooled from three time points. MRI grades show significantly lower in the resveratrol group than the DMSO group (*P = 0.039). (C) Data are presented as mean±SD. |
 | Fig. 3Gene expression analyses of aggrecan and matrix metalloprotease-13 (MMP-13) between the DMSO group and the resveratrol group. (A) Aggrecan mRNA level in resveratrol-treated discs is significantly increased than that in DMSO-treated discs at 16 weeks post treatment (*P = 0.027). (B) MMP-13 mRNA levels significantly is decreased in resveratrol-treated discs than those in DMSO-treated discs at 8 and 16 weeks (†P = 0.006 and ‡P = 0.048, respectively). Data are presented as mean±SD. |
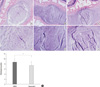 | Fig. 4Histologic analyses of intervertebral disc tissues treated with DMSO and resveratrol. 40 × (A, B, and C) and 100 × (a, b, and c) power images of hematoxylin and eosin staining. Normal disc has a clear demarcation between the nucleus pulposus (NP) and the annulus fibrosus, and abundant extracellular matrix (ECM) (A and a). The degenerated, DMSO-treated disc loses the characteristics. They show indistinct NP margin and severe fibrosis in ECM (B). High power image reveals many fibroblast-like cells, suggesting degeneration (b). In contrast, the resveratrol-treated disc shows the features of restoration. At higher magnification, large, round, grape-like clusters of chondrocytic cells in the NP of the resveratrol-treated disc (c). Significant lower histological grades are noted in the resveratrol group when compared with the DMSO group (*P = 0.024) (D). Data are presented as mean ± SD. |
ACKNOWLEDGMENTS
Hae-Jin Kim PhD, Eun-Joung Moon PhD, Yang-Seon Kim PhD, and Hyeon-Jeong Kim MS at Ensoltek Co., Ltd. provided the technical support and thoughtful comments on the design for in vitro study. The technical support for in vivo studies was provided by the Laboratory Animal Research Center in the Samsung Biomedical Research Institute (SBRI). SBRI is an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) accredited facility and abide by the Institute of Laboratory Animal Resources (ILAR) guide.
Notes
References
1. Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford). 2009; 48:5–10.
2. Freemont TJ, LeMaitre C, Watkins A, Hoyland JA. Degeneration of intervertebral discs: current understanding of cellular and molecular events, and implications for novel therapies. Expert Rev Mol Med. 2001; 2001:1–10.
3. Chujo T, An HS, Akeda K, Miyamoto K, Muehleman C, Attawia M, Andersson G, Masuda K. Effects of growth differentiation factor-5 on the intervertebral disc: in vitro bovine study and in vivo rabbit disc degeneration model study. Spine (Phila Pa 1976). 2006; 31:2909–2917.
4. Hohaus C, Ganey TM, Minkus Y, Meisel HJ. Cell transplantation in lumbar spine disc degeneration disease. Eur Spine J. 2008; 17:492–503.
5. Kandel R, Roberts S, Urban JP. Tissue engineering and the intervertebral disc: the challenges. Eur Spine J. 2008; 17:480–491.
6. Kwon YJ, Lee JW, Moon EJ, Chung YG, Kim OS, Kim HJ. Anabolic effects of Peniel 2000, a peptide that regulates TGF-β1 signaling on intervertebral disc degeneration. Spine (Phila Pa 1976). 2013; 38:E49–E58.
7. Masuda K, Imai Y, Okuma M, Muehleman C, Nakagawa K, Akeda K, Thonar E, Andersson G, An HS. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine (Phila Pa 1976). 2006; 31:742–754.
8. Moon SH, Gilbertson LG, Nishida K, Knaub M, Muzzonigro T, Robbins PD, Evans CH, Kang JD. Human intervertebral disc cells are genetically modifiable by adenovirus-mediated gene transfer: implications for the clinical management of intervertebral disc disorders. Spine (Phila Pa 1976). 2000; 25:2573–2579.
9. Estrov Z, Shishodia S, Faderl S, Harris D, Van Q, Kantarjian HM, Talpaz M, Aggarwal BB. Resveratrol blocks interleukin-1beta-induced activation of the nuclear transcription factor NF-kappaB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood. 2003; 102:987–995.
10. Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000; 164:6509–6519.
11. Sun C, Hu Y, Liu X, Wu T, Wang Y, He W, Wei W. Resveratrol downregulates the constitutional activation of nuclear factor-kappaB in multiple myeloma cells, leading to suppression of proliferation and invasion, arrest of cell cycle, and induction of apoptosis. Cancer Genet Cytogenet. 2006; 165:9–19.
12. Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxford). 2008; 47:809–814.
13. Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005; 7:R732–R745.
14. Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007; 9:R77.
15. Li X, Phillips FM, An HS, Ellman M, Thonar EJ, Wu W, Park D, Im HJ. The action of resveratrol, a phytoestrogen found in grapes, on the intervertebral disc. Spine (Phila Pa 1976). 2008; 33:2586–2595.
16. Wuertz K, Quero L, Sekiguchi M, Klawitter M, Nerlich A, Konno S, Kikuchi S, Boos N. The red wine polyphenol resveratrol shows promising potential for the treatment of nucleus pulposus–mediated pain in vitro and in vivo. Spine (Phila Pa 1976). 2011; 36:E1373–E1384.
17. Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, Andersson GB, An HS. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine (Phila Pa 1976). 2005; 30:5–14.
18. Sobajima S, Kompel JF, Kim JS, Wallach CJ, Robertson DD, Vogt MT, Kang JD, Gilbertson LG. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine (Phila Pa 1976). 2005; 30:15–24.
19. Csaki C, Keshishzadeh N, Fischer K, Shakibaei M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem Pharmacol. 2008; 75:677–687.
20. Shakibaei M, Csaki C, Nebrich S, Mobasheri A. Resveratrol suppresses interleukin-1beta-induced inflammatory signaling and apoptosis in human articular chondrocytes: potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem Pharmacol. 2008; 76:1426–1439.
21. Makarov SS. NF-kappa B in rheumatoid arthritis: a pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res. 2001; 3:200–206.
22. Miagkov AV, Kovalenko DV, Brown CE, Didsbury JR, Cogswell JP, Stimpson SA, Baldwin AS, Makarov SS. NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci U S A. 1998; 95:13859–13864.
23. Wehling N, Palmer GD, Pilapil C, Liu F, Wells JW, Müller PE, Evans CH, Porter RM. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009; 60:801–812.
24. Subbaramaiah K, Chung WJ, Michaluart P, Telang N, Tanabe T, Inoue H, Jang M, Pezzuto JM, Dannenberg AJ. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J Biol Chem. 1998; 273:21875–21882.
25. Elmali N, Esenkaya I, Harma A, Ertem K, Turkoz Y, Mizrak B. Effect of resveratrol in experimental osteoarthritis in rabbits. Inflamm Res. 2005; 54:158–162.
26. Urban JP, McMullin JF. Swelling pressure of the inervertebral disc: influence of proteoglycan and collagen contents. Biorheology. 1985; 22:145–157.
27. Sobajima S, Shimer AL, Chadderdon RC, Kompel JF, Kim JS, Gilbertson LG, Kang JD. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005; 5:14–23.
28. Anderson DG, Izzo MW, Hall DJ, Vaccaro AR, Hilibrand A, Arnold W, Tuan RS, Albert TJ. Comparative gene expression profiling of normal and degenerative discs: analysis of a rabbit annular laceration model. Spine (Phila Pa 1976). 2002; 27:1291–1296.
29. Omlor GW, Lorenz H, Engelleiter K, Richter W, Carstens C, Kroeber MW, Guehring T. Changes in gene expression and protein distribution at different stages of mechanically induced disc degeneration: an in vivo study on the New Zealand white rabbit. J Orthop Res. 2006; 24:385–392.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download