Abstract
Authors evaluated pulmonary tuberculosis (PTB) history as a risk factor for lung cancer in current male smokers in a prospective, population-based cohort study. The subjects were the 7,009 males among the participants in the Seoul Male Cancer Cohort Study for whom there was full information on PTB history and smoking habits. With a 16-yr follow-up, 93 cases of lung cancer occurred over the 99,965 person-years of the study. The estimated relative risk (RR) of PTB history of current smokers in lung cancer after adjusting for three confounders - intake of coffee and tomatoes, and age at entry - was 1.85 (95% CI: 1.08-3.19). The observed joint RRs and attributable risks (ARs) across strata of three confounders were greater than the expected, indicating a positive interaction. Thus a history of PTB in current smokers may be another risk factor for lung cancer. Based on a synergic interaction, a heavy male smoker with a PTB history would be expected to belong to the group at high risk of lung cancer.
Lung cancer was the most commonly diagnosed cancer as well as the leading cause of cancer death in males in 2008 globally (1). While globally, more than a million cancer deaths annually are due to tobacco use (2), lung cancer is the most preventable of all of the major forms of cancers because about 90% of deaths from lung cancer are a result of active cigarette smoking (3).
Recently, Liang et al. (4) reported a significant relationship between a history of pulmonary tuberculosis (PTB) and risk of lung cancer through a systematic review of 31 articles. However, they pointed out that the expected relative risk of lung cancer in PTB patients may be found in the absence of any PTB effect, unless there is proper adjustment for smoking history. So, they suggested a prospective cohort study among non-smokers with a large group of lung cancer cases to minimize the impact of cigarette smoking. However, the increased lung cancer risk observed among never smokers with PTB may be due to confounding by life-time exposure to environmental tobacco smoke (5). To control for the confounding effects of smoking habits, this study aimed to evaluate the relationship between previously medical history of PTB and lung cancer risk in current male smokers through a population-based, prospective cohort design.
The study cohort was from the Seoul Male Cancer Cohort (SMCC), which has been partially reported elsewhere (6-9). The cohort was established in 1992 and 1993 and was designed to investigate the relationship between exposure to lifestyle factors and diet and the risk of major cancers in Korean males. The age distribution of this cohort was similar to that of the Korean population. The 7,009 current smokers from the cohort were selected as the final study participants.
A 15-page confidential questionnaire included questions on demographic characteristics, height and weight, personal and family history of cancer and other diseases including PTB, use of medicines and vitamins, brief physical activity assessments, alcohol and tobacco use, and a food-frequency diet questionnaire addressing usual eating habits over the previous year. The reproducibility and validity of the questionnaire was evaluated in some participants (10).
Follow-up was done over the 16 yr from January 1, 1993, to December 31, 2008. Incident cases of all cancers, including lung cancer, occurring during the 16-yr follow-up period were identified through the database of the Seoul Regional Cancer Registry (SRCR) as a population-based cancer registry (11), the Korea Central Cancer Registry (KCCR) as a nationwide, hospitalbased, cancer registry (12), and death certificates at Statistics Korea (13).
A past medical history of PTB, smoking habits, and potential confounders were obtained using a self-administered questionnaire survey, which was administered in 1992. Subjects were asked to report a medical history of PTB (yes, no). Subjects were classified by duration of smoking (-30, or 31+ yr), daily amount of smoking (-20, or 21+ cigarettes/day), and total cigarette index (TCI), obtained from smoking duration times daily amounts of smoking (-20, or 21+ pack-years).
A potential confounder among the variables of the baseline survey was defined as a variable showing statistical significance, based on a crude relative risk (cRR) or P value of chi-square test for trends in lung cancer. By this definition, the following three potential confounders were chosen: intake of coffee and tomatoes, and age at entry (Table 1).
Age at entry was categorized into 5-yr groups of 40-44, 45-49, 50-54, and 55-59 yr. Frequency of coffee intake was measured using three categories: 0, 1-6, or 7+ cups per week. Information on consumption of tomatoes was collected by semi-quantitative, food frequency methods. After converting to gram equivalents, the covariates with unknown cut-off values were categorized according to quartiles.
Total person-years were calculated by determining the number of days from the start of follow-up, January 1, 1993, until the date of all cancer diagnoses, death from other causes, or the end of follow-up, December 31, 2008, after which the number of days was converted to years. The adjusted relative risk (aRR) of PTB history in current smokers was calculated using Cox proportional hazards regression, adjusting for potential confounders. Confidence intervals were obtained by the Wald method and all reported P values are two-sided. The chi-square test for trends was used to evaluate linear trends.
To assess any interaction of smoking habit and PTB history in the occurrence of lung cancer, two strategies - stratification and a modifier interaction model - were used (14). Stratification by the duration of smoking, amount of daily smoking, and TCI was conducted to check homogeneity of effects in the strata. After confirming homogeneity, comparisons between observed and expected joint effects of PTB history and smoking by multiplicative and addictive interaction models were done. The expected RRs and ARs obtained by multiplicative model and by addictive model respectively were calculated on the basis of their independent effects (15). Analyses were conducted using the STATA software (version 12) (16).
During the total 99,965 person-years of follow-up, 93 newly diagnosed cases of lung cancer were identified in the 7,009 study participants. Table 1 shows the univariate analyses of potential confounders. Based on crude RR and P-values of chi-square trends, age at entry, and intake of coffee and tomatoes were selected to control for confounding effects.
While the prevalence of past medical history of PTB was 9.3% ( = 658/7,009), 93 cases of lung cancer occurred during 16 yr of follow-up in Table 2. The cRR of PTB history in occurring lung cancer was 2.01, and this was statistically significant. Due to the potential influence of smoking on the incidence of lung cancer, smoking habit would represent a very strong confounder in an association between PTB history and lung cancer. To control for it, stratified analyses were conducted using three variables: duration of smoking, amount of daily smoking, and TCI. While there was heterogeneity within each stratum by all three variables, cRRs in the strata of duration of smoking over 31 yr, of daily smoking amounts over 21 cigarettes, and of TCI over 21 pack-years showed positive associations with statistical significance.
The estimated aRR of PTB history in current smokers was 1.85, with statistical significance, after adjusting for age at entry, and intake of tomatoes and coffee in Table 3. The aRRs of each stratum revealed the same results, showing heterogeneity between strata and statistical significance in Table 2.
Because stratification assumes homogeneity within each stratum (14), we evaluated an effect measure modification instead of adjustment. Table 4 summarizes the expected and observed joint effects of PTB history by multiplicative and addictive interaction models. In all three smoking habit-related variables, the observed joint RRs and ARs were greater than the expected, indicating a positive interaction.
Based on these results, PTB history may be a risk factor for lung cancer in current smokers. Additionally, the risk would be intensified by heavier smoking - longer duration of smoking, larger amount of daily smoking, and higher TCI index. These findings indicate the same synergistic interaction and attribution as those in the association between asbestos and lung cancer (17).
While cigarette smoking is well-established as the main cause of lung cancer and about 90% of cases are thought to be tobacco-related (6), only 1%-15% of smokers are eventually diagnosed with lung cancer (18). This indicates that other etiological factors - in conjunction with smoking - cause lung cancer, beyond the purely stochastic nature of the disease process (19). That is, cigarette smoking cannot be the only causative factor in lung cancer, emphasizing the importance of searches for additional etiological and risk factors. These factors include genetics, environmental or occupational exposures, such as to arsenic or radiation, dietary habits, and pulmonary diseases, including tuberculosis (4, 20).
According to a WHO report, an estimated 1.4 million people died from tuberculosis in 2011 (21). As this study showed a 1.85-fold increased risk of lung cancer in current smokers with a medical history of PTB, the level of RR in this study was similar to the summary estimates (RR = 1.97) obtained from studies of 'non-Westernized countries' (4). A higher risk of fatality from lung cancer among patients with active PTB has been reported in one analytic epidemiologic study (22), but not in another (23).
While the precise mechanism of the direct role of tuberculosis remains unclear, some plausible biological mechanisms have been suggested (24). One hypothesis is that pulmonary infection and inflammation may be a cancer initiator and/or a promoter in lung epithelial, by phagocyte-generated oxidants (25) or some proinflammatory cytokines (26). That is, tuberculosis may represent inflammatory processes that augment the effects of other carcinogenic exposures, trap carcinogens in scar tissues, and enhance abnormal cellular growth and proliferation (27). Additionally, inflammation-mediated events, such as the production of reactive oxygen species, the activation of growth factors for wound repair, and the altering of signal transduction processes to activate cell proliferation to replace necrotic/apoptotic tissue cells, are all considered to be components of risk for a variety of cancers (28).
Despite the prospective nature of this study, several sources of bias should be considered when interpreting the results. First, diagnosis of past and/or current PTB as well as smoking habits at cohort entry were based on self reports so that misclassification of exposure, including recall bias, may have occurred. However, the possibility of this bias would be considered to be less than expected because PTB patients in Korea were prescribed long-term, anti-tuberculosis medication mandatorily by public health centers or local clinics so that PTB could be differentiated readily from other lung diseases. Additionally, the results of this prospective cohort study could eliminate the potential for the differential misclassification of exposure better than a case-control study. That is, because the past medical history was ascertained prior to lung cancer occurrence, misclassification of prior PTB would be non-differential, and therefore bias in this study was most likely in a conservative direction (29). However, the lack of details as to the severity, extent, and location of PTB did restrict our inferences, and further studies including these items should be conducted.
Second, a confounding effect by a factor common to both PTB and lung cancer may explain the observed positive association. In particular, smoking habit is so strongly associated with lung cancer and with some other lung diseases, including PTB, that a residual confounding effect is a concern. To overcome any such effect, stratification and effect measure modification using three smoking habit-related variables were evaluated.
Third, the results were obtained from current smokers only. To understand the mechanism of PTB history in occurring lung cancer, follow-up studies in non-smokers with longer follow-up periods would be needed to increase the statistical power; these should control for life-time environmental tobacco smoke exposure (5).
Fourth, another possible non-causal explanation of the observed association is that a common genetic or environmental precursor may lead independently to PTB and lung cancer. Song et al. (30) reported higher abnormal fragile histidine triad (FHIT) protein expression in tumors with tuberculosis. A better understanding of the genetic susceptibility to both PTB and lung cancer, and how these factors may interact with modifiable risk factors in Koreans, is needed.
Finally, there may be lung cancer cases diagnosed as active PTB after starting the follow-up. Although they seem to be few and 16-yr follow-up as the induction period is relatively short, a potential bias by them should be considered. As they were categorized as the non-exposure group in analysis, they might let the estimated results be underestimated.
In summary, our data suggest that a history of PTB is associated with an increased risk of lung cancer in current male smokers, and the risk of PTB history exhibited a synergistic interaction with daily amount, duration of smoking, and TCI, respectively. Therefore, a heavy male smoker with a past medical history of PTB belongs to the group at high risk of lung cancer, and should quit smoking. Additionally, it seems prudent that individuals with PTB should take extra-precautions to reduce exposure to known lung carcinogens, including active smoking.
Figures and Tables
Table 1
Crude relative risks (cRR) and 95% confidence intervals (CI) in occurring lung cancer according to potential confounders in smokers of the Seoul Male Cancer Cohort Study*
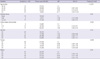
Table 2
Crude relative risks (cRR) and 95% confidence intervals (CI) of a past medical history of pulmonary tuberculosis (TBHX) in occurring lung cancer by smoking habit-related variables in smokers of the Seoul Male Cancer Cohort Study

Table 3
Adjusted relative risks (aRR) and 95% confidence intervals (CI) of past medical history of pulmonary tuberculosis in occurring lung cancer in smokers of the Seoul Male Cancer Cohort Study

Table 4
Relative risks (RR) by multiplicative model and attributable risks (AR) by addictive model with the expected (E) and observed (O) joint effects of past medical history of pulmonary tuberculosis in occurring lung cancer in smokers of the Seoul Male Cancer Cohort Study*

*O, observed; E, expected; NHX, no past medical history of pulmonary tuberculosis; YHX, past medical history of pulmonary tuberculosis; DUR1, duration of smoking ≤ 30 yr; DUR2, duration of smoking ≥ 31 yr; AMT1, amount of daily smoking ≤ 20 cigarettes/day; AMT2, amount of daily smoking ≥ 21 cigarettes/day; TCI1, total cigarette index ≤ 20 pack-years; TCI2, total cigarette index ≥ 21 pack-years; †Per 100,000 person-years.
Notes
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
2. Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M. Comparative Risk Assessment collaborating group (Cancers). Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005; 366:1784–1793.
3. Gazdar AF, Thun MJ. Lung cancer, smoke exposure, and sex. J Clin Oncol. 2007; 25:469–471.
4. Liang HY, Li XL, Yu XS, Guan P, Yin ZH, He QC, Zhou BS. Facts and fiction of the relationship between preexisting tuberculosis and lung cancer risk: a systematic review. Int J Cancer. 2009; 125:2936–2944.
5. Taylor R, Najafi F, Dobson A. Meta-analysis of studies of passive smoking and lung cancer: effects of study type and continent. Int J Epidemiol. 2007; 36:1048–1059.
6. Bae JM, Lee MS, Shin MH, Kim DH, Li ZM, Ahn YO. Cigarette smoking and risk of lung cancer in Korean men: the Seoul Male Cancer Cohort Study. J Korean Med Sci. 2007; 22:508–512.
7. Bae JM, Ahn YO. A nested case-control study on the high-normal blood pressure as a risk factor of hypertension in Korean middle-aged men. J Korean Med Sci. 2002; 17:328–336.
8. Shin MH, Kim DH, Bae JM, Lee HK, Lee MS, Noh JY, Ahn YO. The effect of coffee consumption on serum total cholesterol level in healthy middle-aged men. Korean J Prev Med. 1994; 27:200–216.
9. Kim DS, Koo HW, Kim DH, Bae JM, Shin MH, Lee MS, Lee CM, Ahn YO. A cohort study of physical activity and all cause mortality in middle-aged men in Seoul. Korean J Prev Med. 1998; 31:604–615.
10. Kim MK, Lee SS, Ahn YO. Reproducibility and validity of a self-administered semiquantitative food frequency questionnaire among middle-aged men in Seoul. Korean J Community Nutr. 1996; 1:376–394.
11. Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer incidence in five continents. vol. VIII. Lyon: IARC Scientific Publications;2002. p. 276–277.
12. The Korea Central Cancer Registry in National Cancer Center. Cancer statistics. accessed on 15 January 2013. Available at http://ncc.re.kr/english/infor/kccr.jsp.
13. The Statistics Korea. Annual report on the cause of death statistics. accessed on 15 January 2013. Available at http://www.kostat.go.kr/portal/english/resources/2/4/1/index.static.
14. Szklo M, Nieto FJ. Epidemiology: beyond the basics. 2nd ed. Sudbury: Jones & Bartlett Publishers;2006. p. 183–212.
15. Wang X, Elston RC, Zhu X. The meaning of interaction. Hum Hered. 2010; 70:269–277.
16. StataCorp LP. Data analysis and statistical software. accessed on 15 January 2013. Available at http://www.stata.com.
17. Case BW. Asbestos, smoking, and lung cancer: interaction and attribution. Occup Environ Med. 2006; 63:507–508.
18. Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999; 91:1194–1210.
19. Ahsan H, Thomas DC. Lung cancer etiology: independent and joint effects of genetics, tobacco, and arsenic. JAMA. 2004; 292:3026–3029.
20. Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest. 2003; 123:21S–49S.
21. Word Health Organization. Global tuberculosis report 2012. accessed on 15 January 2013. Available at http://www.who.int/tb/publications/global_report/en/index.html.
22. Brenner AV, Wang Z, Kleinerman RA, Wang L, Zhang S, Metayer C, Chen K, Lei S, Cui H, Lubin JH. Previous pulmonary diseases and risk of lung cancer in Gansu Province, China. Int J Epidemiol. 2001; 30:118–123.
23. Alavanja MC, Brownson RC, Boice JD Jr, Hock E. Preexisting lung disease and lung cancer among nonsmoking women. Am J Epidemiol. 1992; 136:623–632.
24. Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert Rev Anticancer Ther. 2008; 8:605–615.
25. Weitzman SA, Gordon LI. Inflammation and cancer: role of phagocytegenerated oxidants in carcinogenesis. Blood. 1990; 76:655–663.
26. Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007; 117:1175–1183.
27. Wu AH, Fontham ET, Reynolds P, Greenberg RS, Buffler P, Liff J, Boyd P, Henderson BE, Correa P. Previous lung disease and risk of lung cancer among lifetime nonsmoking women in the United States. Am J Epidemiol. 1995; 141:1023–1032.
28. Ardies CM. Inflammation as cause for scar cancers of the lung. Integr Cancer Ther. 2003; 2:238–246.
29. Littman AJ, Thornquist MD, White E, Jackson LA, Goodman GE, Vaughan TL. Prior lung disease and risk of lung cancer in a large prospective study. Cancer Causes Control. 2004; 15:819–827.
30. Song L, Yan W, Deng M, Song S, Zhang J, Zhao T. Aberrations in the fragile histidine triad (FHIT) gene may be involved in lung carcinogenesis in patients with chronic pulmonary tuberculosis. Tumour Biol. 2004; 25:270–275.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download