Abstract
We investigated characteristics associated with the efficacy of dipeptidyl peptidase-4 inhibitors (DPP4i) in Korean patients with type 2 diabetes. We reviewed medical records of 477 patients who had taken sitagliptin or vildagliptin longer than 40 weeks. Response to DPP4i was evaluated with HbA1c change after therapy (ΔHbA1c). The Student's t-test between good responders (GR: ΔHbA1c > 1.0%) and poor responders (PR: ΔHbA1c < 0.5%), a correlation analysis among clinical parameters, and a linear multivariate regression analysis were performed. The mean age was 60 yr, duration of diabetes 11 yr and HbA1c was 8.1%. Baseline fasting plasma glucose (FPG), HbA1c, C-peptide, and creatinine were significantly higher in the GR compared to the PR. Duration of diabetes, FPG, HbA1c, C-peptide and creatinine were significantly correlated with ΔHbA1c. In the multivariate analysis, age (r2 = 0.006), duration of diabetes (r2 = 0.019), HbA1c (r2 = 0.296), and creatinine levels (r2 = 0.024) were independent predictors for the response to DPP4i. Body mass index and insulin resistance were not associated with the response to DPP4i. In conclusion, better response to DPP4i would be expected in Korean patients with type 2 diabetes who have higher baseline HbA1c and creatinine levels with shorter duration of diabetes.
Dipeptidyl peptidase-4 inhibitors (DPP4i) are incretin enhancers which inhibit the degradation of incretin hormones such as glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide. DPP4i treatment lets the incretin hormones stay active to stimulate insulin synthesis and secretion while inhibiting glucagon release from pancreatic islets. There are other mechanisms of incretin action such as regulation of gastric emptying and appetite (1). The Food and Drug Administration approved the first DPP4i in 2006. From then on a lot of clinical studies on it have been published. According to a meta-analysis of randomized controlled trials in adults with type 2 diabetes, at least 12 weeks of DPP4i treatment reduced HbA1c levels about 0.74%, which was noninferior to other hypoglycemic agents (2). Recently, DPP4i became one of second-line regimens after metformin in the treatment of type 2 diabetes in the position statement suggested by the American Diabetes Association and the European Association for the Study of Diabetes (3).
As the previous studies with DPP4i showed different potency in glycemic controls depending on various patient characteristics (2, 4, 5), there seem to be more susceptible patients to DPP4i therapy. However, the factors predictive of the blood glucose lowering effect of DPP4i have not yet been determined clearly. One of the predictors may be racial disparity. Glucagon-like peptide-1 (GLP-1) responses to a mixed meal or oral glucose have been observed different between African Americans and European Americans (6, 7), which can affect the efficacy of DPP4i through GLP-1 response. Efficacy and safety of DPP4i have been demonstrated comparable in Asians to those in Caucasians (8), but some researchers suggested more prominent HbA1c reduction with DPP4i in Japanese than in Caucasian (9). There are a few reports on the clinical factors contributing to the efficacy of Sitagliptin, one of DPP4i, in Asian (10-14), but most of the studies are small-sized and retrospective ones, and some results are conflicting. Furthermore, there was no sufficient evaluation about insulin secretory capacity. According to the action mechanisms of DPP4i, insulin secretory capacity can be an important factor in the prediction of DPP4i efficacy. These studies generally suggested that subjects who had higher baseline HbA1c, lower body mass index (BMI) and shorter duration of diabetes would show a good response to DPP4i, which will be covered further in the discussion session below.
Therefore, in the current study, we investigated clinical factors including insulin secretion and resistance that were associated with response to DPP4i in a large number of patients. Although this is a retrospective study, the results would provide us important perspective to decide which patients would have more beneficial effects with DPP4i.
We reviewed electronic medical records of Seoul National University Hospital from January 2008 to March 2011 to find patients with type 2 diabetes who had taken sitagliptin or vildagliptin longer than 40 weeks continuously. We excluded patients who had been under use of insulin, those who changed other oral anti-diabetic drugs (OAD) just before and during the period of DPP4i use, those who had history of malignancy, pancreatitis, thyroid dysfunction or gastrointestinal surgery except appendectomy and those who were taking immune suppressant agents including glucocorticoids. As a result, we could enroll 477 subjects in the final analysis.
Demographic characteristics, height, weight, duration of diabetes and comorbidities such as chronic diabetic complications, hypertension, dyslipidemia, concomitant medications, and history of operation were reviewed by four physicians. Laboratory data were collected through electronic sorting, including fasting plasma glucose (FPG), postprandial plasma glucose, HbA1c, insulin, C-peptide, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglyceride, aspartate aminotransferase, alanine aminotransferase, creatinine, and urine albumin/creatinine ratio (ACR). Glomerular filtration rate was estimated using the equation from the Modification of Diet in Renal Disease (MDRD) study group (15). BMI was calculated as weight in kilograms divided by height in meters squared. Homeostasis model assessment (HOMA) was used in evaluating insulin resistance (HOMA-IR) and pancreatic β-cell function (HOMA-β) (16). Insulin resistance was also evaluated by the quantitative insulin sensitivity check index (QUICKI) (17). Secretory unit of islet transplantation objects (SUITO) index, which could be applicable to the patients with type 2 diabetes (18), was also calculated to evaluate pancreatic β-cell function.
We assessed the response to DPP4i with the HbA1c change from baseline to after 40 weeks therapy (ΔHbA1c). Good responders (GR) were defined as the subjects whose ΔHbA1c was greater than 1.0% and poor responders (PR) as those whose ΔHbA1c was less than 0.5%. According to other clinical trials investigating DPP4i efficacy in patients with similar baseline HbA1c to our subjects, HbA1c reduction by sitagliptin was about 0.7% (2, 19, 20). Considering the expected efficacy of DPP4i, arbitrary cutoff values of ΔHbA1c for the good and the poor responders were selected as mentioned above. As a result, number of the GR was 170 and that of the PR was 168.
Continuous variables are presented as the mean ± standard deviation. Comparisons of continuous variables between the GR and the PR were performed using the Student's t-test. Sex distribution, hypertension, dyslipidemia, microvascular complication and the use of OAD were analyzed by chi-square test. Sex-adjusted partial correlation analysis with the whole enrolled subjects (n = 477) was performed for a univariate analysis. There were notable data loss in fasting insulin and BMI, and a linear multivariate regression analysis was performed without BMI and the parameters using insulin levels. A level of P < 0.05 was considered statistically significant. All analyses were performed using the SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
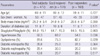
Characteristics of the subjects are demonstrated in Tables 1, 2. Their mean age was 60 yr, BMI was 25.2 kg/m2 and duration of diabetes was 11 yr. Sitagliptin (100 mg/day in 63%, 50 mg/day in 37%) was prescribed to 84.9% of the subjects and vildagliptin (100 mg/day in 32%, 50 mg/day in 68%) to the others. Majority of the subjects was under inadequate glycemic control (baseline HbA1c 8.1 ± 0.9%), and HbA1c reduction after 40-weeks of DPP4i treatment was averaged 0.8 ± 1.1% (Table 2). Other laboratory data including insulin secretion, cholesterol levels and renal function are listed in the Table 2.
Comparisons of baseline characteristics between the GR and the PR demonstrated that there was no difference in age, duration of diabetes, the method of DPP4i use and the rates of diabetic complications. BMI data was available approximately half of the subjects, which were comparable between the two groups. Sex distribution was slightly different between the GR and the PR: more proportion of men was observed in the GR than in the PR (57% vs 45%, P = 0.039, Table 1). The rate of hypertension was higher in the GR, too (68.2% vs 54.8%, P = 0.036).
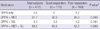
In the Table 2, glucose homeostasis and other laboratory data of the 2 groups are compared. Baseline HbA1c and FPG were significantly higher in the GR (8.5 ± 0.9%; 158 ± 41 mg/dL, respectively) than in the PR (7.7 ± 0.9%; 148 ± 35 mg/dL), but the difference in postprandial plasma glucose was insignificant. After 40-weeks of DPP4i treatment, HbA1c reduced by 1.9 ± 0.7% in the GR, while it rather increased by 0.3 ± 0.6% in the PR, and the percentage reaching HbA1c less than 7% was significantly higher in the GR (67.6%) than in the PR (11.3%). FPG also reduced by 31.5 ± 40.6 mg/dL in the GR, but increased by 15.7 ± 61.4 mg/dL in the PR. Fasting insulin levels and other indices of insulin secretory function and insulin resistance such as HOMA-β, SUITO index, HOMA-IR, and QUICKI were comparable between the groups. However, fasting C-peptide levels were significantly higher in the GR. Fasting insulin levels and HOMA-β could fail of significant difference because of their small number of available data (65% of the subjects in the each group). There were no differences in lipid profiles, aspartate aminotransferase and alanine aminotransferase. Creatinine levels were significantly higher in the GR compared to the PR, while estimated glomerular filtration rate (eGFR) which implied gender effects seemed to be comparable. ACR showed marginal increase in the GR (P = 0.080).
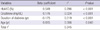
OAD which were concurrently prescribed with DPP4i were not different between the groups (Table 3). Majority of the subjects had taken combination therapy with metformin and sulfonylurea.
According to the sex-adjusted partial correlation analysis, duration of diabetes (r = -0.112, P = 0.025), FPG (r = 0.145, P = 0.002), HbA1c (r = 0.528, P < 0.001), C-peptide (r = 0.127, P = 0.005), and creatinine concentrations (r = 0.116, P = 0.012) were associated with ΔHbA1c (Table 4). Using the ΔHbA1c as a dependent variable, with age, sex, duration of diabetes, HbA1c, C-peptide, creatinine, eGFR, and ACR as independent variables, a multivariate linear regression analysis was performed. We found that age, duration of diabetes, baseline HbA1c, and creatinine levels were independent predictors for the response to DPP4i. All four variables together explained 34.5% of the total variance in ΔHbA1c (Table 5).
Adding a second or third OAD in poorly-controlled old patients with multiple diabetic complications can be challenging, especially when the patients are reluctant to insulin injection. DPP4i are a new therapeutic class for type 2 diabetes that can be used as a monotherapy or an add-on therapy, and may have some advantages over other OAD, particularly for certain high-risk groups: they allow effective glycemic control with low risk of hypoglycemia, neutral effect on body weight, and the convenience of once-daily oral dosing, which may help to improve patients' adherence to therapy (1, 3, 4). However, the cost is relatively high, and it does not work in some patients at all, as we observed in this study. Therefore, we evaluated characteristics of those who showed good response to additive DPP4i, who had not been in adequate glycemic control with metformin and/or sulfonylurea. As far as we know, it is the largest study on the predictors for DPP4i efficacy ever published.
As reported before by previous researchers (10-12), baseline HbA1c was an important predictor for DPP4i efficacy just as other OAD (21). Baseline FPG levels were also observed higher in the GR than in the PR and correlated with ΔHbA1c in the univariate analysis, but it was not an independent predictor in the regression analysis. Postprandial plasma glucose showed no significant difference between the 2 groups nor significant correlation with DPP4i efficacy. Because of the retrospective design of this study, postprandial plasma glucose levels had not been measured by standardized method. Therefore, it is hard to conclude whether the postprandial plasma glucose levels correlate with the response to DPP4i treatment or not in this study. Actually, DPP4i treatment had been reported to improve both fasting and postprandial glycemia (2).
Another independent predictor found in the regression analysis was duration of diabetes. It was also suggested as a predictor in other studies, within the range of 6-11 yr (11-13). Because insulin secretory capacity is known to be decreased according to duration of type 2 diabetes (22), we could speculate that insulin secretory function can be another predictor to DPP4i efficacy. In addition, augmented insulin secretion is one of main mechanisms of DPP4i (1). We evaluated β-cell function using fasting C-peptide (23), HOMA-β (16), and SUITO index (18). Among them, only fasting C-peptide was increased in the GR group compared to the PR (2.5 ± 1.6 vs 2.0 ± 1.2 ng/mL, Table 2), and were associated with ΔHbA1c (r = 0.127, Table 4). However, it lost the statistical significance in the multivariate regression analysis. Therefore, we cannot conclude that good β-cell function is a necessary condition for a good response to DPP4i. There are 2 reports on the relationship between insulin secretion and the response to DPP4i. Lim et al. evaluated factors predicting therapeutic efficacy of combination treatment with sitagliptin and metformin (11). It was a prospective, randomized study in drug-naïve type 2 diabetic patients. They found that high baseline HbA1c and short duration of diabetes were independent parameters for the reduction of HbA1c. However, fasting C-peptide was not different between the highest and the lowest quartiles of HbA1c reduction in the study, while HOMA-β and insulinogenic index were significantly higher in the lowest quartile than in the highest one. Furthermore, insulinogenic index became an independent negative factor for the response to combination therapy. They explained this phenomenon as glucotoxicity: that is, subjects who had higher HbA1c responded better to the combination therapy with sitagliptin and metformin, but they had been under severe glucotoxicity which impaired insulin secretion transiently. They suggested that the low insulinogenic index in the early phase of type 2 diabetes might indicate reversible potential of pancreatic β-cell dysfunction. Under this condition, they suggested that the use of metformin might have reversed the reduced expression of incretin receptor, which would augment the effects of combined sitagliptin. The different study design such as initial combination with sitagliptin and metformin, and the drug-naïve, early phase of type 2 diabetic patients makes it difficult to compare this study to our current one, but both did not support any idea that baseline insulin secretory function is an important predictor to the response to DPP4i. In another study, Kim et al. retrospectively evaluated if baseline insulin secretion as a predictive parameter for the therapeutic efficacy of sitagliptin (10). The subjects were mostly under metformin single therapy, baseline HbA1c was 8.5% and duration of diabetes was 7.7 yr. In that study, fasting C-peptide and HOMA-β were not related with efficacy of sitagliptin in a logistic regression analysis. Therefore, we do not have evidences now about the impact of β-cell function on the DPP4i efficacy. Nonetheless, HOMA index and fasting C-peptide levels provide only an estimate of pancreatic β-cell function, which requires appropriate use and cautious interpretation.
High BMI has been found as an independent predictor for poor response to DPP4i in 2 Japanese studies (12, 13). We could not observe any significant relationship between BMI and DPP4i efficacy in our study. Missing values and non-standardized method of measurement might have caused this discordant result. Lim et al. did not report any relationships between them either, although there was a confounding factor of concurrent combination with metformin (11). If this association exists, the mechanisms why increased BMI is associated with a smaller degree of HbA1c reduction with sitagliptin have yet to be revealed. One possibility is through insulin resistance, because obesity is closely linked to insulin resistance. Insulin resistance was not dealt with by the 2 Japanese researches (12, 13). Our analysis using HOMA-IR and QUICKI did not suggest any relations between insulin resistance and DPP4i efficacy (Tables 2, 4). Alternatively, GLP-1 levels in response to oral intake have been reduced in obese patients, which can attenuate the efficacy of DPP4i as an inhibitor of GLP-1 degradation (24, 25). Further investigations to confirm whether BMI is related to the response to DPP4i and to elucidate the mechanisms would be required.
The DPP4i we examined, sitagliptin and vildagliptin, are excreted mainly by urine (26, 27), and dose adjustments are needed in severe renal impairment (28). In addition, there is a report that dipeptidyl peptidase-4 was exceptionally concentrated in rodent kidney glomeruli (29). We found that creatinine concentrations were significantly higher in the GR compared to the PR (1.00 ± 0.23 vs 0.91 ± 0.19 mg/dL, P < 0.001, Table 2) although they were within normal range. And, the creatinine levels correlated with HbA1c reduction both in the univariate and multivariate analyses (Tables 4, 5). Therefore, trivial differences in glomerular damage or function could have affected the efficacy of DPP4i. However, eGFR estimated by the MDRD equation did not reflect it in the current study. In the study by Bando et al., eGFR was not an independent determinant of HbA1c reduction in response to sitagliptin, either (12). The MDRD equation has poor accuracy when GFR is larger than 60 mL/min/1.73 m2 (30), where the eGFR of the subjects in both the studies lied. According to another study dealing with creatinine levels by Lim et al. (11), response to initial combination of metformin and sitagliptin was not associated with creatinine levels, where we cannot discriminate the effects of metformin. Serum creatinine concentrations are known to be dependent on age, sex, dietary protein and muscle mass, which suggests some confounders other than glomerular function in the interpretation of creatinine levels.
We observed more patients with hypertension or higher ACR levels in the GR compared to the PR (68.2 vs 54.8%; 111.8 ± 292.8 vs 59.7 ± 194.2 mg/g, respectively, Tables 1, 2). We found more patients had both of hypertension and microalbuminuria in the GR compared to the PR (26.5 vs 17.4%, P = 0.049). It suggests that there might be some differences in anti-hypertensive agents, for example angiotensin converting enzyme inhibitors/angiotensin receptor blockers between the groups, and possible drug interaction affecting DPP4i efficacy. Further investigations on this topic would be warranted.
This study has several limitations, because of its retrospectiveness. There are some missing values in several variables, little information on lifestyle and drug compliance, and confounding effects by concurrent medications. Measurement methods of prandial glucose levels and BMI were not strictly standardized. More sophisticated method for evaluating insulin secretion should be applied. In addition, dosage of DPP4i was not standardized in this study. About 42 percent of total subjects received 50 mg/day, which is lower than usual dose (100 mg/day). It was not because of their renal function impairment, but because of the physicians' own decision on optimal treatment. When we excluded these subjects in the analyses, we could observe similar results with weaker statistical significance, which might come from small numbers of subjects. Even though, further prospective study would be needed to confirm our results.
In summary, the present study suggested that high baseline HbA1c and creatinine levels with short duration of diabetes would allow physicians to predict a good response to DPP4i in elderly patients with inadequately controlled type 2 diabetes under metformin with/without sulfonylurea.
Figures and Tables
Table 2
Comparisons of laboratory data between the good responders and the poor responders

Data are presented as mean ± standard deviation or percent (%). *P values by Student's t-test for continuous variables and by chi-square test for frequencies between the good responders and the poor responders; †n = 322 (total), 112 (GR), 111 (PR). eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HOMA-β, homeostasis model assessment of β-cell function; HOMA-IR, homeostasis model assessment of insulin resistance; QUICKI, quantitative insulin sensitivity check index; SUITO, secretory unit of islet transplant objects.
Notes
References
1. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006; 368:1696–1705.
2. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007; 298:194–206.
3. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012; 35:1364–1379.
4. Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP. Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2007; 9:194–205.
5. Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE. Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007; 30:1979–1987.
6. Higgins PB, Férnández JR, Garvey WT, Granger WM, Gower BA. Entero-insular axis and postprandial insulin differences in African American and European American children. Am J Clin Nutr. 2008; 88:1277–1283.
7. Velasquez-Mieyer PA, Cowan PA, Umpierrez GE, Lustig RH, Cashion AK, Burghen GA. Racial differences in glucagon-like peptide-1 (GLP-1) concentrations and insulin dynamics during oral glucose tolerance test in obese subjects. Int J Obes Relat Metab Disord. 2003; 27:1359–1364.
8. Mu YM, Misra A, Adam JM, Chan SP, Chow FC, Cunanan EC, Deerochanawong C, Jang HC, Khue NT, Sheu WH, et al. Managing diabetes in Asia: overcoming obstacles and the role of DPP-IV inhibitors. Diabetes Res Clin Pract. 2012; 95:179–188.
9. Kikuchi M, Abe N, Kato M, Terao S, Mimori N, Tachibana H. Vildagliptin dose-dependently improves glycemic control in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2009; 83:233–240.
10. Kim SA, Shim WH, Lee EH, Lee YM, Beom SH, Kim ES, Yoo JS, Nam JS, Cho MH, Park JS, et al. Predictive clinical parameters for the therapeutic efficacy of sitagliptin in korean type 2 diabetes mellitus. Diabetes Metab J. 2011; 35:159–165.
11. Lim S, An JH, Shin H, Khang AR, Lee Y, Ahn HY, Yoon JW, Kang SM, Choi SH, Cho YM, et al. Factors predicting therapeutic efficacy of combination treatment with sitagliptin and metformin in type 2 diabetic patients: the COSMETIC study. Clin Endocrinol (Oxf). 2012; 77:215–223.
12. Bando Y, Kanehara H, Aoki K, Hisada A, Toya D, Tanaka N. Obesity may attenuate the HbA1c-lowering effect of sitagliptin in Japanese type 2 diabetic patients. J Diabetes Investig. 2012; 3:170–174.
13. Nomiyama T, Akehi Y, Takenoshita H, Nagaishi R, Terawaki Y, Nagasako H, Kudo T, Kodera T, Kobayashi K, Urata H, et al. Contributing factors related to efficacy of the dipeptidyl peptidase-4 inhibitor sitagliptin in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2012; 95:e27–e28.
14. Kim WJ, Park CY, Jeong EH, Seo JY, Seol JS, Park SE, Rhee EJ, Lee WY, Oh KW, Park SW, et al. Retrospective analysis on the efficacy, safety and treatment failure group of sitagliptin for mean 10-month duration. Diabetes Metab J. 2011; 35:290–297.
15. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130:461–470.
16. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–419.
17. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000; 85:2402–2410.
18. Yamada Y, Fukuda K, Fujimoto S, Hosokawa M, Tsukiyama K, Nagashima K, Fukushima M, Suzuki H, Toyoda K, Sassa M, et al. SUIT, secretory units of islets in transplantation: An index for therapeutic management of islet transplanted patients and its application to type 2 diabetes. Diabetes Res Clin Pract. 2006; 74:222–226.
19. Charbonnel B, Karasik A, Liu J, Wu M, Meininger G. Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006; 29:2638–2643.
20. Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P. Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006; 28:1556–1568.
21. Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC. The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis. Diabetes Care. 2010; 33:1859–1864.
22. U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease: U.K. Prospective Diabetes Study Group. Diabetes. 1995; 44:1249–1258.
23. Faber OK, Binder C. C-peptide response to glucagon: a test for the residual beta-cell function in diabetes mellitus. Diabetes. 1977; 26:605–610.
24. Muscelli E, Mari A, Casolaro A, Camastra S, Seghieri G, Gastaldelli A, Holst JJ, Ferrannini E. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes. 2008; 57:1340–1348.
25. Ranganath LR, Beety JM, Morgan LM, Wright JW, Howland R, Marks V. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut. 1996; 38:916–919.
26. Herman GA, Stevens C, Van Dyck K, Bergman A, Yi B, De Smet M, Snyder K, Hilliard D, Tanen M, Tanaka W, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebocontrolled studies with single oral doses. Clin Pharmacol Ther. 2005; 78:675–688.
27. He YL, Sabo R, Campestrini J, Wang Y, Riviere GJ, Nielsen JC, Rosenberg M, Ligueros-Saylan M, Howard D, Dole WP. The effect of age, gender, and body mass index on the pharmacokinetics and pharmacodynamics of vildagliptin in healthy volunteers. Br J Clin Pharmacol. 2008; 65:338–346.
28. Bergman AJ, Cote J, Yi B, Marbury T, Swan SK, Smith W, Gottesdiener K, Wagner J, Herman GA. Effect of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidyl peptidase-4 inhibitor. Diabetes Care. 2007; 30:1862–1864.
29. Kettmann U, Humbel B, Holzhausen HJ. Ultrastructural localization of dipeptidylpeptidase IV in the glomerulum of the rat kidney. Acta Histochem. 1992; 92:225–227.
30. Julie Lin BMD. Azotemia and urinary abnormalities. In : Longo D, Fauci A, Kasper D, Hauser S, Loscalzo J, editors. Harrison's Principles of Internal Medicine. 18th ed. New York: McGraw-Hill;2011.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download