Abstract
The acquisition of metastasis potential is a critical point for malignant tumors. Melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24) is a potential tumor suppress gene and frequently down-regulated in malignant tumors. It has been implicated that overexpression of MDA-7 led to proliferation inhibition in many types of human tumor. Invasion is an important process which is potential to promote tumor metastasis. However, the role and potential molecular mechanism of mda-7/IL-24 to inhibit the invasion of human melanoma cancer is not fully clear. In this report, we identified a solid role for mda-7/IL-24 in invasion inhibition of human melanoma cancer LiBr cells, including decreasing of adhesion and invasion in vitro, blocking cell cycle, down-regulating the expression of ICAM-1, MMP-2/9, CDK1, the phosphorylation of ERK and Akt, NF-κB and AP-1 transcription activity. Meanwhile, there was an increased expression of PTEN in mda-7/IL-24 over-expression LiBr cells. Our results demonstrated that mda-7/IL-24 is a potential invasion suppress gene, which inhibits the invasion of LiBr cells by the down-regulation of ICAM-1, MMP-2/9, PTEN, and CDK1 expression. The molecular pathways involved were the MAPK/ERK, PI3K-Akt, NF-κB, and AP-1. These findings suggest that mda-7/IL-24 may be used as a possible therapeutic strategy for human melanoma cancer.
Melanoma is a malignant tumor of melanocytes. It is the 19th most common cancer worldwide, estimated to be responsible for almost 200,000 new cases of cancer in 2008 (more than 1% of the total) (1). Although melanoma is less common than other skin cancers, it is much more dangerous if it is not found early. It causes the majority (75%) of deaths related to skin cancer (2). During the past 3 decades, the incidence, morbidity, and mortality of malignant melanoma have increased dramatically (3). Surgery is the first choice of treatment for primary melanoma. However, adjuvant treatments are needed for patients with high risk of recurrence and late staged disease (4). Recent advance in the understanding of the molecular alterations and immunoregulatory processes in melanoma have led to new treatment approaches that have been approved for clinical use. These include the IL-2 plus vaccine (5), Ipilimumab, a monoclonal antibody against CTLA-4 (cytotoxic T lymphocyte antigen-4) which activates T cells to defend the tumor (6), and Vemurafenib, which is an inhibitor of the BRAF kinase with V600E mutation, a common mutation in melanoma (7). Moreover, melanoma differentiation associated gene-7 (mda-7) is another promising target that is now in clinical trial development. The mda-7 was initially identified using a subtraction hybridization approach from cDNA libraries isolated from human melanoma cells induced to terminally differentiate by interferon-β and mezerin (8). Subsequence studies led to the classification of MDA-7 as a member of the IL-10 cytokine family and designation it as IL-24 (9). Ellerhorst et al. showed that the MDA-7 protein is produced in human melanocytes and in primary melanomas but its expression is progressively lost in metastatic melanomas (10). The anti-proliferation efficacy of MDA-7 in human melanoma and other cancer was first demonstrated by Jiang et al. (11). Subsequent studies provided consistent evidence that ectopic expression of mda-7 resulted in apoptosis induction and cell death in a wide type of solid tumors, including melanoma, malignant glioma, breast cancer, lung cancer and etc). Based on these impressive antitumor properties in preclinical researches, a replication incompetent adenovirus expressing mda-7 gene (Ad. mda-7; INGN 241) has got into clinical trial to evaluate its antitumor potency in human (12). The mechanisms of mda-7 to inhibit tumor are rather complicated. It has been shown that mda-7 induces apoptosis by altering diverse signaling pathways in tumor cells, plays as a regulator of toxic autophagy, overcomes chemo-resistance in multiple cancers when combined with other anti-cancer drugs, and also exists a potent "bystander antitumor" activity in pancreatic carcinoma.
It has been shown that ectopic production of mda-7/IL-24 inhibits invasion and migration of human lung cancer cells and the mechanism is not well understood. Though this gene was first found in the melanoma, the anti-invasion activity of mda-7/IL-24 in melanoma cells has not yet been well investigated. In the present study, we demonstrated that over-expression of MDA-7/IL-24 inhibited the invasion of melanoma cells and several molecular pathways were involved.
The human melanoma cell line LiBr was supplied by the Department of Immunology of Tianjin Cancer Hospital. The cells were maintained at 37℃ in a humidified atmosphere with 5% CO2. The culture medium was RPMI-1640 supplemented with 10% fetal bovine serum (FBS). Cells were detached by 0.25% trypsin-0.53mM EDTA and sub-cultured routinely.
The pCI-neo mammalian expression vector was purchased from Promega. This plasmid vector carries the human cytomegalovirus (CMV) immediate-early enhancer/promoter region to promote constitutive expression of cloned DNA inserts in mammalian cells. The full-length of mda-7 cDNA was isolated and prepared as described before (11). The cDNA was inserted into the multiple cloning region of the vector according to the instruction manual. The recombinant plasmids were amplified in Escherichia coli. The plasmids were harvested and the presence of the mda-7 insert was checked by restrictive digestion and DNA electrophoresis.
The transfection was performed by using the Transfectam® Reagent (Promega) according to the instruction manual. Briefly, LiBr cells were allowed to reach about 60%-70% confluency in 60-mm dishes. Immediately before transfection, gently washed the cells in serum-free medium and added 0.5 mL of serum-free medium per dish. Added 5 µg control or recombinant plasmid DNA to 500 µL of serum-free medium (Solution A), then added 20 µL of Transfectam® Reagent (Solution B). Mixed solution A and B and added directly to the cells and incubated the cells with solution overnight. At the end of the incubation period, added 4 mL of complete medium and returned the cells to the incubator for another 24 hr. Then cells were plated in 6-well plates with selection medium, which contained 600 µg/mL G418. This concentration of G418 was chose from pre-studies and was the lowest concentration that could kill the non-transfected cells within 7 days. Successfully transfected cell clones were obtained by 2 weeks culture in the selection medium and the expression of MDA-7 was assessed by Western blotting.
Cells invasion was assessed by a model based on the CytoSelect 24-well cell invasion assay kit (8 µm pore size, Cell Biolabs) according to the manufacturer's instructions. Briefly, cells were harvested by trypsinization and washed by phosphate-buffered saline (PBS) and suspended in serum-free medium at 5 × 106 cells/mL. Add 500 µL of media containing 10% FBS to the lower well of the invasion plate, and 300 µL of the cell suspension solution to the inside of each insert. Plates were incubated for 48 hr at 37℃ in cell incubator. Gently swab the interior of the inserts to remove non-migratory cells. Migrated cells on the lower surface of the filter was stained and counted with a light microscope.
Adhesion of cells to ECM was studied using the CytoSelect 48-well cell adhesion assay kit (Cell Biolabs) according to the manufacturer's instructions. Briefly, cells were serum-starved overnight, detached by trypsinization and allowed to express novel integrins in the shaking cell incubator in complete medium containing 1% bovine serum albumin (BSA). Added 5 × 105 cells in 150 µL complete medium to the inside of each well and incubated for 2 hr in cell incubator. Discarded the medium and washed the wells 4 times by PBS. The adhering cells was stained and determined by a fluorescence plate reader at 480 nm/520 nm.
Transfected and parental LiBr cells were cultured in complete medium to reach 70%-80% confluency in 6-well plates. Cells were eliminated by trypsinization and proteins from the ECM were solubilized in TNC buffer (50 mM Tris-HCl, pH 7.5, 0.15 M NaCl, 10 mM CaCl2 and 0.05% Brij-35) and then centrifuged at 15,000 g for 5 min. To get the whole proteins, the collected cells were lysed by RAPI lysis buffer (Millipore) and then centrifuged at 12,000 g for 10 min. Total protein concentrations of the supernatants were measured by the BCA method (Sigma Aldrich). Proteins (10 µg) were separated on 12% SDS-PAGE and transferred onto PVDF membranes. Membranes were blocked for 2 hr at room temperature in TNT buffer (10 mM Tris-HCl and 150 mM NaCl pH 7.4, 0.1% Tween-20) with 5% non-fat dried milk, incubated overnight at 4℃ with rabbit anti-MDA-7/IL24, total-ERK, p-ERK, total-Akt, p-Akt, CDK1, and β-actin antibody (Santa Cruz) or with goat anti-MMP-2, MMP-9, ICAM-1 and PTEN (Santa Cruz). All primary antibodies were diluted according to the instruction manual. Membranes were washed and incubated for 1 hr with peroxidase-labelled anti-rabbit IgG or anti-goat IgG (Santa Cruz, diluted at 1:2,000). Finally, membranes were washed three times in TNT and exposed to the Immobilon™ Western chemiluminescent HRP substrate (Millipore) for 1 min, and then exposed autoradiography film for 1-5 min in the dark.
Cells were cultured and more than 106 cells were harvested. The total mRNA was extracted from the harvested cells using the Dynabeads mRNA Direct Kit (Invitrogen) according to the manufacturer's instruction manual. Total mRNA was then reverse transcribed for 1 hr at 42℃ in incubation buffer containing 250 µM of each deoxynucleotide triphosphate, 5 µM oligo (dT)20, 25 units of RNase inhibitor, and 20 units of avian myeloblastosis virus reverse transcriptase (Roche Diagnostics). The transcription level of MMP-2/-9, ICAM-1, CDK1, and PTEN were detected by semiquantitative real-time PCR using the icycler iQ detection system (Bio-Rad) with prepared primers (Table 1). The PCR condition was as following: decontamination at 50℃ for 2 min, denaturation at 95℃ for 2 min, followed by n cycles at 95℃ for 20 sec and at hybridization T℃ for 40 sec.
Cells were cultured in 10-cm culture dishes and allowed to reached -70% confluency. Then cells were harvested by trypsinization, fixed with 75% ethanol at -20℃ for 2 hr, and then resuspended in 500 µL of a propidium iodide (PI) solution (Sigma) (5 µg/mL PI and 10 µg/mL RNase). DNA contents and cell cycle phases were analyzed using BD FACSCalibur flow cytometer.
Cells were cultured in complete culture medium to reach -75% confluency. Harvested the cells by trypsinization and resuspended the cells in complete culture medium. Seeded about 8 × 103 viable cells into 96-well plate in triplicate and incubated overnight to get the cells adhesion. Transfected each well of cells with 0.1 µg pGL 4.32 [luc2P/AP-1-RE/Hypro] and [luc2P/NF-κB-RE/Hypro] plasmids (Promega) according to the instruction manual. These plasmids contain five copies of NF-κB and AP-1 response element that drives transcription of the luciferase reporter gene luc2P (Photinus pyralis). After 24 hr of transfection, the luciferase activity was analyzed by Bright-Glo™ Luciferase Assay System (Promega).
Human melanoma cell line LiBr was transfected with recombinant plasmids encoding mda-7 gene (pCI-Neo-mda-7) or with negative control plasmid (pCI-Neo). Two pCI-Neo-mda-7 (clone 1 and 2) and one control plasmid transfected cell clones were selected from G418 resistance assay. The total cellular proteins were extracted from the parental (non-transfected), clone 1 and 2, and negative control clone of LiBr cells. The expression of MDA-7 was determined by western blotting. As shown in Fig. 1, MDA-7 was weakly expressed in the parental and control cells, but strongly expressed in clone 1, and to a higher extent in clone 2 cells.
To investigate the effect of MDA-7 on the cells metastasis, we determined whether the invasion ability of the cells through matrigel, which mimics the metastatic process of tumor cells to travel through basement membrane components, was inhibited. The invasion ability of negative control cells was unaffected when compared to the parental cells. However, the number of cells that transferring though the matrigel were significantly reduced in clone 1 and 2 by 33.3% and 15.9% respectively, as compared to the parental cells (Fig. 2). To further investigate the potential inhibition of MDA-7 on tumor cell metastasis, we determined whether cell attachment to ECM components was affected by overexpression of MDA-7. As shown in Fig. 3, the adhesion ability was unchanged to negative control cells, but was inhibited significantly in clone 1 (54.1%) and 2 (46.5%) cells, as compared to the parental cells. We noticed that, both invasion and adhesion abilities of clone 2 were weaker than clone 1, which represented a reverse relationship with the MDA-7 protein expression level.
We noticed that the mda-7 transfected cells had a moderately slower growth rate (data not shown). Therefore, we would like to investigate if the cell cycle was arrest by MDA-7 expression and to some extent resulted in the inhibition of invasion. The cells was stained by PI, and analyzed by flow cytometer. As shown in Fig. 4, the cells from clone 1 and 2 were tend to arrested at G2/M phase, as a higher percentage of cells were present at this phase, when compared to the control and parental cells.
To investigate the molecular mechanisms involved in the negative effects of MDA-7 overexpression on tumor cell invasion and adhesion, the expression of invasion- and adhesion-related molecules, such as MMP-2, -9, CDK1, PTEN and ICAM-1, were determined by Western blotting in pCI-NEO-mda-7 transfected LiBr cells. As shown in Fig. 5A, the MMP-2, -9, CDK1 and ICAM-1 were significantly downregulated in mda-7 transfected clone 1 and 2 cells and PTEN was significantly upregulated in mda-7 overexpressing cells. RT-PCR showed that the gene transcription of these molecules was attenuated (Fig. 5B).
As MDA-7 could modulate the expression of the molecules mentioned above, the possibly involved signaling pathways were investigated. We determined the ERK and Akt phosphorylation by western blotting and the transcriptional activity of NF-κB and AP-1 by reporter gene assay system. As shown in Fig. 6, the phosphorylation of ERK and Akt was significantly decreased in clone 1 cells, and to a more extent in clone 2 cells. Similarly, the overexpression of MDA-7 in tumor cells was accompanied by downregulation of NF-κB and AP-1 transcriptional activity, as shown in Fig. 7 by the fluorescence activity of the reporter gene luciferase.
It has been shown that mda-7 is a tumor suppressor gene in a various kinds of cancer including melanoma, lung carcinoma, and breast carcinoma. MDA-7 extends its anti-cancer efficacy by inhibiting proliferation, inducing apoptosis, increasing the sensitivity of tumor cells to chemotherapy, and anti-metastasis and so on (13). However, the mechanism of MDA-7 to prevent the tumor from matastasis is not well investigated. Here, we shown that MDA-7 could reduce the invasion of LiBr melanoma cells, and this regression of invasion was accompanied by modulation of several metastasis-related molecules and pathways.
We chose the human melanoma cell line LiBr as our research tool because it has been reported that LiBr is a highly malignant and proliferative tumor cell line (14) and our in-lab studies have confirmed it as well. The LiBr cells did express low level of MDA-7 protein and we suspected that there had some relationship between the low expression of this protein and the high malignance property of the cells. We reconstructed LiBr by transfecting mda-7-encoding plasmids into the cells and obtained two cell clones which stably over-expressed MDA-7. The expression of MDA-7 in the clonal cells were validated by western blotting which showed that clone 2 had a higher expression level of MDA-7 than clone 1. This difference in expression conferred an advantage to the two clones to further investigate whether the follow-up effects were dose-dependent. We first investigated the influence of MDA-7 overexpression on in vitro tumor cells' invasion through matrigel and adhesion to ECM, which represented two important processes of in vivo tumor meatstasis. The results strongly suggested that MDA-7 decreased the invasion and adhesion of the cells.
It has been shown that MDA-7 has direct anti-tumor potency in several tumors, and we also observed that the growth rate was moderately decreased in mda-7 transfected cells (data not shown). To figure out whether the inhibition of cell migration was due to the MDA-7 mediated cell proliferation arrest, we analyzed the cell cycle by flow cytometer. We observed that the number of cells in G2/M phase was increased in clone 1 and 2, compared to the control cells. We concluded that the inhibition of MDA-7 on cell invasion was partly, but not completely, due to the inhibition of proliferation, as the former effect was more obvious and comprehensive than the latter one. The inhibition of tumor metastasis was induced by an arrest, in most cases, in G2/M phase and because CDK1 are essential in initiating and coordinating the cell division cycle phases, we hypothesized that the inhibition of cell cycle arrest induced by MDA-7 may be linked to their action on CDK1. In this study, we investigated whether MDA-7 alters CDK1 expression while blocked cell cycle. We present evidence that a G2/M phase cell cycle block is induced through MDA-7 involving direct inhibition of the CDK1 expression. Our studies suggest that the inhibition of CDK1 expression may play an important role in MDA-7 induced metastasis inhibition.
Cancer cell invasion involved the degradation of the ECM. MMP-2 and -9 are two important members in the metalloproteinase superfamily. It has been shown that they are closely related to tumor invasion since their high expression could help the cancer cells to travel through the ECM powerfully (15). We found that the MMP-2 and -9 were downregulated in clone 1 and 2 cells. These findings were correlative to the inhibition of cell invasion by MDA-7. Since other MMPs are also involved in the invasion, we could not exclude their involvement in the modulation of invasion by MDA-7. This possibility needs to be further investigated.
The alteration of adhesive interaction of cancer cells to the surrounding cells is another important step in tumor metastasis. ICAM-1 is a member of the immunoglobulin superfamily, which is typically expressed on the surface of endothelial cells to mediate the transmigration of leukocytes into the tissues (16). In the epithelial derived carcinoma, such as melanoma, the expression of ICAM-1 is correlated to the malignancy of the diseases (17). Moreover it has been reported that ICAM-1 was involved in cellular adhesion and promoted metastasis of melanoma cells in vitro (18). Based on these reports, we investigated the effect of MDA-7 on ICAM-1 expression. Western blotting showed that the ICAM-1 level was decreased in clone 1 and 2 cells and it was further confirmed by RT-PCR determination.
The mechanisms by which MDA-7 downregulated MMP-2/9, CDK1 and ICAM-1 in LiBr cell were not clear. The clear thing is that, these modulations of protein expression are largely due to the modulation on transcription level, as shown by RT-PCR. Tumor invasion and metastasis have previously been shown to be regulated by numerous molecular pathways, including the MAPK/ERK and PI3K-Akt pathway (19, 20). Moreover, it has been reported that MDA-7 shapes the cancer cell behavior by a complicate intracellular pathways, including MAPK/ERK and PI3K-Akt pathway as well (21). Another common tumor suppressor gene, PTEN, deserves to be paid attention to. PTEN can block FAK, PI3K, and Akt signaling (22). Upregulation of PTEN by MDA-7 has been demonstrated in breast, lung tumor cells (23) and glioma cells (24). The downstream events of MAPK/ERK and PTEN/PI3K/Akt pathways are multiple gene expression. Indeed, these pathways are involved in MMP-2/9 production in some instant (25, 26). Our results showed that the phosphorylation of ERK was decreased in clone 1 and 2 cells, and in contrast, the expression of PTEN was upregulated, which means PI3K/Akt pathway could be inhibited and the phosphorylation of Akt was decreased. However, the underlying modulation sequences might be more complicate than obvious. One example has been shown by Ahmed Hamaï A et al. (27). They observed that the PTEN/PI3K/Akt pathway could be modulated by knockdown of ICAM-1. In conclusion, our in vitro studies showed that mda-7 was an anti-invasion gene in human melanoma. Overexpreesion of MDA-7 inhibited invasion and adhesion potency of LiBr cells. This inhibition was correlated with expression downregulation of MMP-2, -9 and ICAM-1, which are relative to tumor metastasis and progression. The mechanism of these proteins expression modulation by MDA-7 might be through pathways of MAPK/ERK, PTEN/PI3K/Akt and NF-κB or AP-1. The details of mechanism need to be further validated. All of these modulations were happened in a dose-dependent manner to MDA-7 expression.
Figures and Tables
 | Fig. 1The construction of stably MDA-7 overexpression LiBr cell lines. Western blot showed increased MDA-7 expression in MDA-7 overexpressing LiBr cells. β-actin was used as an internal control for loading. The experiment shown is representative of three independent experiments with similar results. |
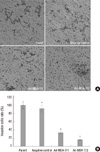 | Fig. 2Effects of MDA-7 on cell invasion of LiBr cell lines in vitro. (A) The invasion assays of LiBr cells were measured by determined cell counts and photographed at × 20 magnification through transwell chambers. (B) The graph showed decreased invasion cells in MDA-7 overexpressing LiBr cells. Bars mean±SD. *P > 0.05, †P < 0.05, n=3. |
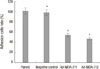 | Fig. 3Effects of MDA-7 on cell adhesion of LiBr cell lines in vitro. The in vitro adhesion was measured by determined by MTS assay. The results showed decreased adherent cells in MDA-7 overexpressing LiBr cells. Bars mean±SD. *P > 0.05, †P < 0.05, n=5. |
 | Fig. 4Effects of MDA-7 on cell cycle of LiBr cell lines in vitro. (A) The cell cycle of LiBr cells was measured by flow cytometry. (B) The graph showed a significant arrest in the C2/M phase in MDA-7 overexpressing LiBr cells. Bars mean±SD. *P > 0.05, †P < 0.05, n=3. |
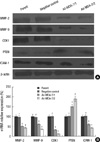 | Fig. 5Effects of MDA-7 on expression of MMP-2/9, CDK1, ICAM-1 and PTEN in LiBr cell lines. (A) Western blot showed decreased MMP-2/9, CDK1 and ICAM-1 expression and increased PTEN expression on protein level in MDA-7 overexpressing LiBr cells. β-actin was used as an internal control for loading. The experiment shown is representative of three independent experiments with similar results. (B) Real-time PCR showed decreased MMP-2/9, CDK1 and ICAM-1 expression and increased PTEN expression on mRNA level in MDA-7 overexpressing LiBr cells. GAPDH was used as an internal control for loading. Bars mean±SD. *P > 0.05, †P < 0.05, n=5. |
 | Fig. 6Effects of MDA-7 on phosphorylation of ERK and Akt in LiBr cell lines. Western blot showed decreased phosphorylation of ERK and Akt in MDA-7 overexpressing LiBr cells. β-actin was used as an internal control for loading. The experiment shown is representative of three independent experiments with similar results. |
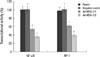 | Fig. 7Effects of MDA-7 on NF-κB and AP-1 transcriptional activation of LiBr cell lines. The transcriptional activation was detected by luciferase reporter assay. The results showed decreased transcriptional activation of NF-κB and AP-1 in MDA-7 overexpressing LiBr cells. Bars mean ± SD. *P > 0.05, †P < 0.05, n=5. |
References
1. Soerjomataram I, Lortet-Tieulent J, Parkin DM, Ferlay J, Mathers C, Forman D, Bray F. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012. 380:1840–1850.
2. Turrisi R, Gunn H, Hultgren B, Warner N, Mallett KA. The style project: feasibility of collaborating with salons for prevention and early detection of skin cancer. Arch Dermatol. 2012. 148:1206–1207.
3. Lutzky J. New therapeutic options in the medical management of advanced melanoma. Semin Cutan Med Surg. 2010. 29:249–257.
4. Allan C, Smithers BM. Surgery and the management of cutaneous melanoma. Br J Surg. 2013. 100:313–315.
5. Smith FO, Downey SG, Klapper JA, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Restifo NP, Levy CL, et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res. 2008. 14:5610–5618.
6. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010. 363:711–723.
7. Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011. 364:2507–2516.
8. Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995. 11:2477–2486.
9. Emdad L, Lebedeva IV, Su ZZ, Gupta P, Sauane M, Dash R, Grant S, Dent P, Curiel DT, Sarkar D, et al. Historical perspective and recent insights into our understanding of the molecular and biochemical basis of the antitumor properties of mda-7/IL-24. Cancer Biol Ther. 2009. 8:391–400.
10. Fisher PB, Sarkar D, Lebedeva IV, Emdad L, Gupta P, Sauane M, Su ZZ, Grant S, Dent P, Curiel DT, et al. Melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24): novel gene therapeutic for metastatic melanoma. Toxicol Appl Pharmacol. 2007. 224:300–307.
11. Wang CJ, Zhang H, Chen K, Zheng JW, Xiao CW, Ji WW, Yu Y, Hu HY, Li Y, Xue XB. Ad.mda-7 (IL-24) selectively induces apoptosis in hepatocellular carcinoma cell lines, suppresses metastasis, and enhances the effect of doxorubicin on xenograft tumors. Oncol Res. 2010. 18:561–574.
12. Eager R, Harle L, Nemunaitis J. Ad-MDA-7; INGN 241: a review of preclinical and clinical experience. Expert Opin Biol Ther. 2008. 8:1633–1643.
13. Gaud G, Iochmann S, Guillon-Munos A, Brillet B, Petiot S, Seigneuret F, Touzé A, Heuzé-Vourc'h N, Courty Y, Lerondel S, et al. TFPI-2 silencing increases tumour progression and promotes metalloproteinase 1 and 3 induction through tumour-stromal cell interactions. J Cell Mol Med. 2011. 15:196–208.
14. Sun B, Zhang S, Ni C, Zhang D, Liu Y, Zhang W, Zhao X, Zhao C, Shi M. Correlation between melanoma angiogenesis and the mesenchymal stem cells and endothelial progenitor cells derived from bone marrow. Stem Cells Dev. 2005. 14:292–298.
15. Herszényi L, Hritz I, Lakatos G, Varga MZ, Tulassay Z. The behavior of matrix metalloproteinases and their inhibitors in colorectal cancer. Int J Mol Sci. 2012. 13:13240–13263.
16. Lydka M, Bilinska B, Cheng CY, Mruk DD. Tumor necrosis factor α-mediated restructuring of the Sertoli cell barrier in vitro involves matrix metalloprotease 9 (MMP9), membrane-bound intercellular adhesion molecule-1 (ICAM-1) and the actin cytoskeleton. Spermatogenesis. 2012. 2:294–303.
17. Parfiniewicz B, Pendzich J, Gruchlik A, Hollek A, Weglarz L. Impact of celecoxib on soluble intercellular adhesion molecule-1 and soluble e-cadherin concentrations in human colon cancer cell line cultures exposed to phytic acid and TNF-alpha. Acta Pol Pharm. 2012. 69:1283–1290.
18. Zhang P, Ozdemir T, Chung CY, Robertson GP, Dong C. Sequential binding of αVβ3 and ICAM-1 determines fibrin-mediated melanoma capture and stable adhesion to CD11b/CD18 on neutrophils. J Immunol. 2011. 186:242–254.
19. Sun X, Essalmani R, Day R, Khatib AM, Seidah NG, Prat A. Proprotein convertase subtilisin/kexin type 9 deficiency reduces melanoma metastasis in liver. Neoplasia. 2012. 14:1122–1131.
20. Marchesi F, Piemonti L, Mantovani A, Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010. 21:77–82.
21. Dent P, Yacoub A, Hamed HA, Park MA, Dash R, Bhutia SK, Sarkar D, Wang XY, Gupta P, Emdad L, et al. The development of MDA-7/IL-24 as a cancer therapeutic. Pharmacol Ther. 2010. 128:375–384.
22. Bonavida B, Baritaki S. The novel role of Yin Yang 1 in the regulation of epithelial to mesenchymal transition in cancer via the dysregulated NF-κB/Snail/YY1/RKIP/PTEN Circuitry. Crit Rev Oncog. 2011. 16:211–226.
23. Eulitt PJ, Park MA, Hossein H, Cruikshanks N, Yang C, Dmitriev IP, Yacoub A, Curiel DT, Fisher PB, Dent P. Enhancing mda-7/IL-24 therapy in renal carcinoma cells by inhibiting multiple protective signaling pathways using sorafenib and by Ad.5/3 gene delivery. Cancer Biol Ther. 2010. 10:1290–1305.
24. Hamed HA, Yacoub A, Park MA, Eulitt PJ, Dash R, Sarkar D, Dmitriev IP, Lesniak MS, Shah K, Grant S, et al. Inhibition of multiple protective signaling pathways and Ad.5/3 delivery enhances mda-7/IL-24 therapy of malignant glioma. Mol Ther. 2010. 18:1130–1142.
25. Adya R, Tan BK, Punn A, Chen J, Randeva HS. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc Res. 2008. 78:356–365.
26. Chen JS, Wang Q, Fu XH, Huang XH, Chen XL, Cao LQ, Chen LZ, Tan HX, Li W, Bi J, et al. Involvement of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma: association with MMP-9. Hepatol Res. 2009. 39:177–186.
27. Hamaï A, Meslin F, Benlalam H, Jalil A, Mehrpour M, Faure F, Lecluse Y, Vielh P, Avril MF, Robert C, et al. ICAM-1 has a critical role in the regulation of metastatic melanoma tumor susceptibility to CTL lysis by interfering with PI3K/AKT pathway. Cancer Res. 2008. 68:9854–9864.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download