Abstract
Efficient management of human tissue samples is a critical issue; the supply of samples is unable to satisfy the current demands for research. Lack of informed consent is also an ethical problem. One of the goals of the 2012 revision of Korea's Bioethics and Safety Act was to implement regulations that govern the management of human tissue samples. To remain competitive, medical institutions must prepare for these future changes. In this report, we review two tissue management models that are currently in use; model 1 is the most common system utilized by hospitals in Korea and model 2 is implemented by some of the larger institutions. We also propose three alternative models that offer advantages over the systems currently in use. Model 3 is a multi-bank model that protects the independence of physicians and pathologists. Model 4 utilizes a comprehensive single bioresource bank; although in this case, the pathologists gain control of the samples, which may make it difficult to implement. Model 5, which employs a bioresource utilization steering committee (BUSC), is viable to implement and still maintains the advantages of Model 4. To comply with the upcoming law, we suggest that physicians and pathologists in an institution should collaborate to choose one of the improved models of tissue management system that best fits for their situation.
The successful completion of the Human Genome Project led modern medical science into a new era of personalized medicine. Human tissue specimens are essential for translational research in personalized medicine (1) and extremely large data sets must be generated before investigators are able to proceed with clinical trials. Because of the increasing demands for human tissue, efficient management of tissue specimens has become a critical and important issue for pathology departments (2-4).
Archived human tissue is in short supply and the current levels cannot satisfy the demand for research. Although many hospitals store vast amounts of tissue samples, informed consent has not been collected in most cases (5); therefore, these samples cannot be used for research purposes. The paucity of high quality tissue specimens and the lack of certified bioresource banks are additional problems that contribute to the limited supply of materials for modern research.
One of the goals of the 2012 revision of Korea's Bioethics and Safety Act was to design regulations that govern the management of human biological material, including tissue samples. This law stipulates physicians to obtain informed consent when collecting human tissue samples for research and clarifies the processes for proper collection, storage, and distribution of specimens (6). Because this law requires clinicians to obtain informed consent prior to collecting tissue samples, many of the ethical issues surrounding the use of these samples for research will be resolved and access to existing tissue samples for research purposes will be enhanced. Many hospitals are expected to implement changes to their tissue management systems in order to take advantage of the benefits of the upcoming law.
Hospital pathology departments process every tissue sample that is collected and store the vast majority of archived samples (7, 8). In addition, the pathology departments are legally responsible for management of these samples (9). Therefore, it would be impossible to implement any changes to the way in which clinical tissue archives are tracked and managed without the enthusiastic support of the pathology departments themselves.
Here, we describe five different human tissue management models. The purpose of this report is to highlight the advantages and challenges associated with each model, which will be useful for policymakers trying to determine the most appropriate management model for specific hospitals. The first and second models are currently used by hospitals in Korea; the third, fourth, and fifth models are suggested improved systems for the management of human tissue samples.
The scope of this paper is limited to human tissue samples used for research; it does not include a description of management models for tissues used for therapeutic transplantation, or liquid human biological material, such as urine, blood, and sweat.
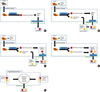
In this model, which is operated by many pathology departments, the raw tissue samples are collected by clinicians and sent to the pathology laboratory for processing or analysis. The pathologist examines, selects, and processes the specimens into formalin-fixed, paraffin-embedded (FFPE) tissue samples and the FFPE samples are archived for future diagnosis and research. Following approval by the hospital's institutional review board (IRB), the pathologist supplies the requested human tissue samples to the researcher (Fig. 1A, Table 1).
The advantages of this model are that it does not require the use of a certified bioresource bank, and management of the pathology FFPE archive for research purposes requires no additional maintenance costs (Table 2). However, this model has many limitations. First, the pathologist can only supply researchers with FFPE tissue samples; the absence of a formal bioresource bank makes it impossible to store fresh or frozen tissue, cell lines, or high quality DNA or RNA samples, which can only be obtained from fresh tissue (Table 2). Second, the use of archived FFPE tissue samples is ethically questionable because informed consent is typically not obtained by the clinician. In most cases, use of archived FFPE tissue samples for research purposes would require the researcher to obtain the informed consent of the specific patient in compliance with the Bioethics and Safety Act.
In this model, the clinician obtains informed consent from the patient to allow storage of the tissue in the hospital's government-certified bioresource bank. The sample is then sent to the pathology laboratory, where a portion of the fresh tissue is processed into FFPE samples and archived, and another portion is sent to the bioresource bank by the pathologist (Fig. 1B, Table 1). A technician at the bioresource bank extracts DNA, RNA, and protein, or cultivates cell lines from the fresh tissue sample. In this model, the use of specimens stored in the hospital's bioresource bank for research purposes does not require IRB approval; therefore, researchers can easily obtain high quality DNA, RNA, and protein samples, as well as isolated cell lines (Table 2). However, as in model 1, if a researcher requires access to the FFPE tissue archive, the informed consent of the patient and IRB approval must be obtained.
It should be noted that the revised Bioethics and Safety Act will allow the use of fresh tissue samples to be exempted from an IRB review (10). From a research perspective, model 2 has an advantage over model 1 because it provides researchers with convenient access to high quality DNA and RNA samples that are essential to modern medical research. On the other hand, model 2 would require hospitals to establish government-certified bioresource banks, which may not be economically or logistically feasible for smaller hospitals or those without sufficient resources (Table 2).
This model is similar to model 2 in that the clinician obtains informed consent from the patient to allow storage of the tissue in government-certified bioresource banks, and fresh tissue samples are processed into cell lines, nucleic acid and protein samples at the hospital's bioresource bank. However, in this model, the pathology department operates its own post-diagnosis FFPE tissue bank; only tissue samples that have already been used for diagnosis may be stored in the FFPE tissue bank (Fig. 1C, Table 1). The remaining tissue is archived so that it may be used for future diagnosis. Access to FFPE tissue samples does not require IRB approval and researchers can simply submit a request to the pathology department itself. However, distribution of tissue from the archive to the FFPE tissue bank remains a controversial ethical issue. For example, archived tissue samples may be required in cases of cancer recurrence and when new medical breakthroughs occur; the type and number of samples that should be archived is a question faced by pathologists on a daily basis.
Since informed consent has been obtained for the samples in both bioresource banks, this model allows researchers to access a large number of human tissue specimens without the need for IRB approval. However, the costs associated with establishing and operating multiple bioresource banks may be a burden that only a few hospitals can afford (Table 2).
Model 4 combines the functions of the two bioresource banks described in model 3 into a single bank that stores and processes both FFPE and fresh tissue samples and is managed by the pathology department (Fig. 1D, Table 1). Implementation of this model would streamline the storage process, reduce operational costs, and simplify the application process when accessing samples for research purposes (Table 2).
Although this model is the most efficient method of managing tissue samples for research, physicians are likely to feel uncomfortable with the idea of assigning complete control of the samples to the pathology department. To alleviate these concerns, a system could be established in which the physician who obtained informed consent from the patient is given control of the samples. Although the initial opposition to model 4 by physicians may be strong, serious consideration should be given to ideas that can accommodate a single bioresource bank.
This model is an alternative to model 4; instead of providing complete control of the FFPE and fresh tissue samples to the pathology department, a hospital bioresource bank would be established and its operation would be supervised by a BUSC (Fig. 1E, Table 1). The advantage of this model is similar to that of model 4, in that access to clinical samples for research purposes would be simplified (Table 2). The disadvantages of model 5 include the costs and resources associated with establishment of the BUSC, and pathologists' concerns over loss of control of the samples (Table 2). However, if the BUSC is composed of multiple individuals that balance the viewpoints of physicians, pathologists, and researchers, these groups would be more inclined to share their tissue sample archives. This balance would be essential for generating the trust that is necessary for pathology departments to actively participate in the system. Whereas the goal of model 4 was to alleviate the concerns of physicians, the main objective of model 5 is to alleviate the concerns of the pathology department. A system in which the pathologists are given ample opportunities to collaborate with various researchers could be established. Because most pathology departments control over 90% of human clinical samples, the success of model 5 is critically dependent on the enthusiastic support of these departments.
Model 1 represents the most common system utilized by hospitals in Korea. Its limitations are well known; it is inconvenient for researchers to access existing samples, high quality samples are scarce, and use of these samples for research purposes is ethically questionable. Overall, this model is outdated and hospitals using this model must implement changes to remain competitive. Model 2 represents a system that has now been implemented by some of the larger hospitals in Korea. The certified bioresource bank provides researchers with high quality tissue samples that are essential to modern medical research. However, this model fails to sufficiently utilize the vast FFPE archive of the pathology department. Hospitals using this model must find a way to distribute the FFPE samples into a certified bioresource bank. Models 3, 4, and 5 represent proposals to modernize tissue management processes.
Model 3 allows hospitals to create and maintain a large database of tissue samples through the use of dual or multiple government-certified hospital bioresource banks. However, operating more than one bank would be inconvenient for researchers and expensive for the hospital. Despite these inefficiencies, model 3 would be the most convenient system to implement in medical institutions. Physicians and pathologists would be able to retain control over their samples and hospital administrators could avoid political friction. It is for these reasons that some large medical institutions may be considering implementation of this model.
Among the models described in this report, model 4 is the most efficient. In this model, every sample is managed by a single bioresource bank in a single department, which creates a huge database and enables efficient and cost-effective management of tissue samples. However, most physicians are unwilling to relinquish control of the samples to the pathology department. Attempts to implement this system would undoubtedly face fierce opposition from physicians. Since it may be impractical to implement this model in a large medical institution, formation of a feasible system that maintains the advantages of model 4 is desirable.
Model 5 is proposed as a viable alternative to model 4. By relinquishing control over the hospital bioresource bank to the BUSC, it may be possible to reach a political compromise while still maintaining the benefits of model 4. For model 5 to succeed, physicians and pathologists must reach a compromise that will ultimately benefit both themselves and the wider research and clinical communities.
Transition from models 1 or 2 to models 3, 4, 5, or other innovative models is an issue that every medical institution in Korea will face in the near future. Each medical institution faces challenges that are unique to their situation. The physicians and pathologists in these institutions are ultimately responsible for conceptualizing and implementing the solutions.
Figures and Tables
Fig. 1
Schematic drawings of models. (A) Model 1: Human tissue sample archive managed by the pathology department. (B) Model 2: Government-certified bioresource bank managed by the hospital. (C) Model 3: Dual government-certified bioresource banks, managed independently by the hospital and the pathology department. (D) Model 4: Single government-certified hospital bioresource bank managed by the pathology department that stores both fresh and FFPE tissue samples. (E) Model 5: Single government-certified comprehensive hospital bioresource bank operated by the bioresource utilization steering committee.
References
1. Hewitt RE. Biobanking: the foundation of personalized medicine. Curr Opin Oncol. 2011. 23:112–119.
2. Riegman PH, van Veen EB. Biobanking residual tissues. Hum Genet. 2011. 130:357–368.
3. Giannini C, Oelkers MM, Edwards WD, Aubry MC, Muncil MM, Mohamud KH, Sandleback SG, Nowak JM, Bridgeman A, Brown ME, et al. Maintaining clinical tissue archives and supporting human research: challenges and solutions. Arch Pathol Lab Med. 2011. 135:347–353.
4. Ryu YJ, Kim H, Jang S. Proposal for the development of a human biological material management system for research hospitals. J Korean Med Assoc. 2012. 55:292–303.
5. Gefenas E, Dranseika V, Serepkaite J, Cekanauskaite A, Caenazzo L, Gordijn B, Pegoraro R, Yuko E. Turning residual human biological materials into research collections: playing with consent. J Med Ethics. 2012. 38:351–355.
6. Baik SJ, Park O, Kim YJ. Summary of bioethics and safty act: bioethics forum. accessed on 1 March 2013. Available at http://www.nibp.kr/xe/2117.
7. Bevilacqua G, Bosman F, Dassesse T, Höfler H, Janin A, Langer R, Larsimont D, Morente MM, Riegman P, Schirmacher P, et al. The role of the pathologist in tissue banking: European Consensus Expert Group Report. Virchows Arch. 2010. 456:449–454.
8. Stephenson J. Pathologists enter debate on consent for genetic research on stored tissue. JAMA. 1996. 275:503–504.
9. Medical Service Act of Korea. No.10387: article 22. accessed on 1 March 2013. Available at http://www.law.go.kr/main.html.
10. Bioethics and Safety Act of Korea. No.11250: article 36②: enforcement rule: article 33①. accessed on 1 March 2013. Available at http://www.law.go.kr/main.html.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download