Abstract
A 14-month-old boy was transferred because of dilated and hypertrophied left ventricle, neutropenia, and developmental delay. After checking computed tomographic angiography with contrast-dye, the patient showed acute exacerbation and finally died from multi-organ failure despite intensive cares. From genetic analysis, we revealed that the patient had Barth syndrome and found a novel hemizygous frame shift mutation in his TAZ gene, c.227delC (p.Pro76LeufsX7), which was inherited from his mother. Herein, we report a patient with Barth syndrome who had a novel mutation in TAZ gene and experienced unexpected acute exacerbation after contrast dye injection for computed tomographic angiography.
Barth syndrome is a rare X-linked recessive disorder presenting from infancy, with characteristic symptoms of cardiomyopathy, neutropenia, skeletal myopathy, growth retardation, and 3-methylglutaconic aciduria (1, 2). It is the first reported inborn error associated with cardiolipin dysfunction induced by mutations in the tafazzin (TAZ) gene at Xq28. Cardiolipin is an inner mitochondrial membrane lipid with an essential role in the stability of the mitochondrial respiratory chain (3). Herein, we report a case of a novel mutation of the TAZ gene in Barth syndrome in a young child who showed acute exacerbation after contrast-dye injection and died from multiorgan failure.
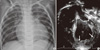
A 14-month-old boy was referred to our hospital from another hospital because of dilated and hypertrophied left ventricle (LV), neutropenia, and developmental delay on 27 October 2011. He was born at full term, with a body weight of 3.2 kg. Sixteen days after birth, he was hospitalized owing to persistent irritability. A chest radiograph showed cardiomegaly, and an echocardiogram revealed decreased LV contractility (ejection fraction: 24%). Under the impression of myocarditis, he had been managed for 1 yr before presentation in our hospital. Moreover, the patient showed feeding difficulty and developmental delay from birth. Before referral, he had been admitted to other hospitals 7 times because of infection episodes. He was taking furosemide, spironolactone, enalapril, and carvedilol before referral. When he was referred to our hospital at 14 months old, his body weight was 6 kg (less than 3rd percentile) and his height was 71 cm (less than 3rd percentile). His overall motor development was delayed, and he could not sit alone. He could say "mama" and "papa." The recorded blood pressure and heart rate were 94/30 mmHg and 132 beats per minute, respectively. On physical examination, no definite heart murmur was audible and the liver was not palpable. He also showed persistent neutropenia, which started during his stay in the previous hospital. His WBC and neutrophil counts were 8,800/µL and only 2% (176/µL), respectively. The B-natriuretic peptide level was 1,045 pg/mL. A chest radiograph showed mild cardiomegaly (cardiothoracic ratio: 62.8%; Fig. 1A), and an electrocardiogram showed a low QRS voltage at the limb leads. An echocardiogram revealed a dilated and hypertrophied globular LV with a hypertrophied papillary muscle and hyper-trabeculation, which did not meet the criteria of LV non-compaction. The other echocardiographic parameters were as follows: LV internal diameter at diastole, 37.7 mm (Z = 10.2); ejection fraction, 36.6%; and LV mass index, 75.6 g (Z = 6.3; Fig. 1B). To rule out the systemic cause of the dilated and hypertrophied LV, we performed a thoraco-abdominal computed tomographic (CT) angiography with contrast dye. The CT findings showed no abnormality in the kidney and other organs and vessels. However, after undergoing CT angiography, the patient showed abrupt high-grade spiking fever (Fig. 2) and developed secretory diarrhea (800 cc per day) associated with metabolic acidosis. At this time, his WBC and neutrophil counts decreased to 3,290/µL and 1% (33/µL), respectively. The B-natriuretic peptide level was greater than 4,901 pg/mL. He did not show associated respiratory symptoms. The results of the respiratory and gastrointestinal viral studies were negative, and the blood and stool cultures were negative for pathogens. The C-reactive protein level was 7.08 mg/dL. Despite supportive care including intravenous fluid resuscitation and empirical antibiotics, the patient's condition worsened, with aggravated metabolic acidosis and respiratory difficulty, requiring transfer to the intensive care unit (ICU). Just before the ICU transfer, the serum pH was 6.881; bicarbonate level, 7.8 mmol/L; and total CO2 was 41.4 mm Hg. Although the patient had been treated with intensive ventilator care, several inotropic agents, and other supportive care measures, he eventually died from the aggravated metabolic acidosis and acutely decompensated heart failure 7 days after the ICU care. During the stay in ICU, we performed genetic analysis for Barth syndrome from the evidence of displayed cardiomyopathy, neutropenia, and developmental delay. The gene sequence analysis revealed that his TAZ gene harbored a novel hemizygous frameshift mutation, c.227delC (p.Pro76LeufsX7), which he inherited from his mother (Fig. 3).
Barth syndrome is a very rare X-linked recessive disorder with various types of cardiomyopathy as one of its characteristic symptoms. In this case, we found a novel mutation of the TAZ gene and experienced an unexpected acute exacerbation after contrast dye injection for CT angiography. Since the discovery of Barth syndrome by Barth in 1983, more than 100 different mutations of the TAZ gene have been reported in all exons of Xq28 (4). The level and composition of cardiolipin are affected by the TAZ gene mutation, resulting in the instability of the mitochondrial respiratory chain in the inner mitochondrial membrane lipid (3). This pathological mechanism provokes various expressions of cardiomyopathy, neutropenia, skeletal myopathy, and growth retardation (2). Various types of cardiomyopathy have been reported, including dilated cardiomyopathy, hypertrophic cardiomyopathy, endocardial fibroelastosis, and isolated non-compaction of the ventricular myocardium (5). Spencer et al. (5) reported that approximately half of their patients showed prominent LV trabeculations and 1 patient had hypertrophic CMP, although most patients had dilated CMP. Our patient also showed a dilated and hypertrophied globular LV with prominent trabeculations.
From the abnormal shape of the LV, neutropenia, growth retardation, and developmental delay, we suspected Barth syndrome, which was confirmed by genetic analysis after the patient died. This is the first genetically confirmed case of Barth syndrome in Korea. There might have been many patients who had been treated and died under the impression of dilated CMP or non-compacted LV without definite diagnosis until now in Korea.
In our case, we experienced a sudden unexpected exacerbation of the patient's condition with a high-grade fever, massive diarrhea, associated acute decompensated heart failure, and eventually death after the patient underwent CT angiography with iodinated contrast dye. Because the patient developed an acute high-grade fever and diarrhea and the blood and stool cultures were negative for pathogens during the treatment course, we assumed that the intravascular injection of iodinated contrast dye induced cellular damage by neutrophil and cardiac muscle apoptosis and finally exacerbated the patient's condition. Fanning et al. (6) demonstrated that iodinated contrast media induce neutrophil apoptosis through a mitochondrial and caspase-mediated pathway in vitro study using human neutrophil and provided insights of the pro-apoptotic mechanism of iodinated contrast dye in other cells. Our patient also showed decreased WBC and neutrophil counts 1 day after the contrast dye injection. Nevertheless, it is very difficult to conclude the exact pathophysiological mechanism underlying the acute exacerbation of the patient's condition.
It is interesting that some patients with Barth syndrome show improved cardiac and motor functions after meticulous supportive care as they grow older (7), although infantile Barth syndrome shows a high mortality rate from severe heart failure and bacterial infections. To improve the prognoses of patients with Barth syndrome, physicians should be suspicious of Barth syndrome in patients with dilated cardiomyopathy (especially associated with increased LV hypertrophy including hyper-trabeculations), neutropenia, growth retardation, and weakness in skeletal muscle.
In conclusion, we found a novel mutation of the TAZ gene and experienced unexpected acute exacerbation after contrast dye injection for CT angiography.
Figures and Tables
Fig. 1
Chest radiograph and transthoracic echocardiogram at diagnosis. (A) Chest radiograph showing mild cardiomegaly (cardiothoracic ratio: 62.8%). (B) On echocardiography, the patient showed a dilated and hypertrophied globular left ventricle (LV) with hypertrophied papillary muscle and hyper-trabeculation (arrows).
References
1. Barth PG, Scholte HR, Berden JA, Van der Klei-Van Moorsel JM, Luyt-Houwen IE, Van't Veer-Korthof ET, Van der Harten JJ, Sobotka-Plojhar MA. An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J Neurol Sci. 1983. 62:327–355.
2. Kelley RI, Cheatham JP, Clark BJ, Nigro MA, Powell BR, Sherwood GW, Sladky JT, Swisher WP. X-linked dilated cardiomyopathy with neutropenia, growth retardation, and 3-methylglutaconic aciduria. J Pediatr. 1991. 119:738–747.
3. Barth PG, Valianpour F, Bowen VM, Lam J, Duran M, Vaz FM, Wanders RJ. X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): an update. Am J Med Genet A. 2004. 126A:349–354.
4. Takeda A, Sudo A, Yamada M, Yamazawa H, Izumi G, Nishino I, Ariga T. Eponym: Barth syndrome. Eur J Pediatr. 2011. 170:1365–1367.
5. Spencer CT, Bryant RM, Day J, Gonzalez IL, Colan SD, Thompson WR, Berthy J, Redfearn SP, Byrne BJ. Cardiac and clinical phenotype in Barth syndrome. Pediatrics. 2006. 118:e337–e346.
6. Fanning NF, Manning BJ, Buckley J, Redmond HP. Iodinated contrast media induce neutrophil apoptosis through a mitochondrial and caspase mediated pathway. Br J Radiol. 2002. 75:861–873.
7. Schlame M, Ren M. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 2006. 580:5450–5455.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download