Abstract
This study aimed to investigate the clinical implication of surgical resection for the malignancies of heart and great vessels. Between January 2001 and May 2011, a retrospective review of the results in 12 patients was conducted. There were 6 patients with primary cardiac tumor including leiomyosarcoma, angiosarcoma, undifferentiated type sarcoma and malignant fibrous histiocytoma. The remaining 6 patients had the metastatic tumors or adjacent invasion to the heart and great vessels. Six of seven patients who underwent complete resection had no evidence of recurrence. However, four of five patients who underwent incomplete resection or biopsy showed local recurrence or distant metastasis of residual tumor, and one of them required reoperation for recurred tumor. In-hospital mortality was 8.3% and the mean survival of all patients was 22.2 ± 6.1 months. Survival of the incomplete resection group, except for the two biopsy cases, was 25.9 ± 7.9 months, and there was no mortality in the complete resection group. Therefore, clinical outcomes in patients who had malignancies of the heart and great vessels may be improved when the aggressive and complete resection, or possible debulking for palliation, was performed. Moreover, adjuvant multimodality therapy may be imperative to prevent recurrence or metastasis, and to provide improved survival.
Malignant tumors of the heart and great vessels are very rare. Despite most of the primary cardiac tumors are benign and 75% of those are myxoma (1), primary cardiac sarcomas are the second most common type of primary cardiac neoplasm (2) and metastases to the heart and great vessels are far more common.
The clinical presentation is usually asymptomatic, until the malignancies cause heart dysfunction or metastasize to other organs, and depends on tumor location and size. Patients are often presented with progressive dyspnea and edema due to congestive heart failure (3), and occasionally, syncope with anemia (4) or pericardial tamponade (5, 6), and symptoms due to embolic event or metastasis to other sites.
Treatment of malignancies of the heart and great vessels tumors can be challengeable and the prognosis is poor due to their aggressive behavior and delayed diagnosis. Adding to the difficulty in complete resection of the tumors in these locations, the patients with cardiac surgery under cardiopulmonary bypass was subjected to more risk factors related to the surgery itself than other surgical procedures. However, complete or en bloc surgical resection remains the cornerstone for the treatment of malignancies despite the high incidence of unresectable cases and other risk factors (7). In a few reports, combined heart and lung orthotopic transplantation for curative surgical approach has been attempted in highly selected patients with locally advanced primary cardiac sarcomas (8). Neo-adjuvant or adjuvant chemotherapy and radiation have been used in the recent treatment era. Since there was a lack of previous studies to determine the role of chemotherapy and radiation in malignant cardiac tumors, the clinical impacts have been still controversial. However, in the previous report on multimodality treatment for cardiac sarcoma, there showed that adjuvant treatment for local recurrence or metastatic disease enhanced survival (9). In the prognosis, the median survival of primary heart and great vessel sarcomas has been reported to be 6 months (10) and 17 months (11) during follow up periods, and disease free survival in the current report was 21 months for all cases and 15 months for high-grade tumors during mean the 45 months of the follow up periods (12).
Although clinical outcomes in patients who underwent surgical resection for primary cardiac sarcoma have been investigated, there has been lacked description of the metastatic tumors and malignant fibrous histiocytoma in presenting symptoms, surgical results and follow up information. In the present study, we identified 12 patients with malignant tumors of the heart and great vessels, which we tried to remove by surgery for curative or debulking intent, and also analyzed the clinical implication of the surgical treatment of the tumor.
Between January 2001 and May 2011, twelve patients with malignant tumors of the heart and great vessels were identified and the medical records of these patients were reviewed. Detailed data on patient's basal characteristics, including presenting symptoms, tumor location and histology, resection type, intensive care unit (ICU) and hospital length of day, postoperative complication, response to adjuvant treatment, recurrence during follow up period, and survival were obtained.
Computed tomography (CT) angiogram and echocardiography were used for the initial diagnosis, including concomitant functional and structural abnormalities, and to determine surgical resectability. Cardiac magnetic resonance imaging (MRI) was helpful preoperatively to identify more specific structures of tumors and adjacent invasions, and also used for post-operative monitoring of patients with intraluminal abnormalities on residual tissue as the differentiation of tumor degree and myxoid content, according to the degree of enhancement.
Statistical analysis was performed using the SPSS software package (Version 12.0, SPSS Inc, Chicago, IL, USA). Continuous variables were expressed as the mean ± standard deviation, median and ranges, or proportions. Survival rates were estimated using the Kaplan-Meier method. A P value of less than 0.05 was considered statistically significant.
There were 6 males and females, with the mean age of 53.6 ± 14.1 yr (range 31-79 yr). Clinical symptoms included dyspnea, blood tinged sputum or hemoptysis, chest discomfort, shoulder pain and weight loss. There were no signs and symptoms in 3 patients. Of those, cardiac involvement of metastatic tumor was detected on a follow up CT angiogram in 2 patients who had previous surgical resection of colon cancer and osteosarcoma of the ankle.
In preoperative echocardiography, the mean left ventricle ejection fraction and pulmonary artery systolic pressure was 62.5% ± 6.2% and 45.1 ± 19.2 mmHg, respectively. The most common location of tumor was the left atrium (LA) in 7 patients. Other sites included the right atrium, left ventricle, pericardium, ascending aorta and aortic arch, subclavian and common carotid artery, superior vena cava (SVC) and lung. Except 2 patients who were planned with surgery for tissue diagnosis and palliative debulking, all patients underwent tumor resection for curative intent. The baseline patient characteristics are presented in Table 1.
Cardiopulmonary bypass (CPB) and cardiac arrest was required in the majority of patients (9 of 12) for tumor resection. The mean CPB time and aorta cross clamp (ACC) time were 177.8 ± 67.7 min and 98.3 ± 59.5 min. One patient underwent complete tumor resection and reconstruction of the left subclavian artery and common carotid artery without CPB. Other 2 patients underwent only exploration and biopsy because of direct invasion to the left ventricular free wall and extensive involvement of all cardiac valves and pericardium. However, the central aim of the surgery in these patients had been the debulking of tumor mass for palliation. There was no patient with simple complete excision or palliative debulking of mass without reconstruction or combined surgery. Reconstruction of atrial chambers including interatrial septum, or great vessels were performed with glutaraldehyde (GA)-fixed autologous pericardium in 5, bovine pericardium only in 1, combined use of autologous pericardium and bovine pericardium only in 1 patient. Two patients had the concomitant mitral ring annuloplasty or replacement for tumor involvement of the mitral valve with mitral steno-insufficiency. In two patients with metastatic tumor originated from colon and lung cancer which involved the LA and right lung (Fig. 1A), bilobectomy of the right middle and lower lobe of the lung was performed, via intrapericardial approach as a combined surgery (Fig. 1B). Moreover, one patient with mediastinal germ cell tumor, which extended to the ascending aorta, aortic arch and SVC (Fig. 1C), underwent en bloc resection of tumors, replacement of the total arch and separate replacement of arch vessels using Spielvogel technique (13), and SVC reconstruction with autologous pericardium (Fig. 1D). Detailed information of operative procedures performed was described in Table 2.
Complete resection (R0) was performed in 7 patients. Incomplete resection (R2) was performed in 3 patients due to the invasion of atrioventricular (AV) node (n = 1) or coronary sinus just around to LA inferior wall (n = 1) or both mitral valve annulus and whole LA appendage (n = 1).
The final pathologic diagnoses were listed in Table 3. There were 6 patients with distant metastasis or adjacent invasion from primary lung cancer (n = 2), colon cancer (n = 1), mediastinal germ cell tumor (n = 1), chondrosarcoma (n = 1) and thymic carcinoma (n = 1). In addition, there were 6 patients with primary cardiac malignancies, including 4 sarcomas and two malignant fibrous histiocysomas. The types of sarcoma were leiomyosarcoma (n = 2), angiosarcoma (n = 1) and undifferentiated pleomorphic sarcoma (n = 1).
In the histologic grade of primary cardiac malignancies, considering tumor differentiation, mitotic count and tumor necrosis, according to the Fédération Nationale des Centres de Lutte Contre le Cancer Grading System (FNCLCC Grading), all patients had moderate to high grade of tumor histology. It was presented in 4 cases of moderate grade and 2 cases of high grade.
In-hospital mortality was 8.3% (1 of 12 patients). The patient underwent emergency operation for exploration and resection of large, mobile vegetation on the aortic valve. However, the extensive cardiac and pericardial metastasis of thymic carcinoma was found, thus, only tumor biopsy was performed. The patient died of heart failure with intractable ventricular arrhythmia 18 days after surgery. There was no life threatening morbidities in other patients. The mean length of stay in the intensive care unit was 4.2 ± 4.9 days (range 1-18 days), and the mean hospital length of stay was 16.2 ± 7.4 days (range 7-30 days). There were several minor complications, including atrial fibrillation, which converted to sinus rhythm after amiodarone therapy, pericardial effusion, azotemia and urinary tract infection. Postoperative pneumonia or chylothorax requiring prolonged chest tube drainage occurred in each of the 2 patients, who had undergone bilobectomy of lung as a combined surgery. One patient experienced partial seizure of the right arm, caused by a small amount of subdural hematoma, which seems to have occurred after surgery.
There was no patient with R1 resection. Among the seven patients who underwent complete tumor resection (R0), 5 showed no evidence of local recurrence and distant metastasis. However, two patients with R0 had distant metastasis to the lung and brain during the follow up period. On the other hand, 4 of the five patients who underwent the incomplete tumor resection (R2) or biopsy had residual tumor growth or local recurrence with or without metastases to the other organs. One of them required reoperation for recurred tumor at 2 yr after the first operation. Reoperation was also performed for palliative tumor debulking. Only one patient with malignant fibrous hitiocytoma, who underwent R2 resection, did not show the evidence of disease progression during 11 months after surgery.
Seven patients received adjuvant chemotherapy and 3 patients refused further treatment after surgery. Radiation therapy was added in cases of brain metastasis. The common chemotherapy agents used in our center were etoposide, ifosfamide, mesna, cisplatin and adriamycin. Since the main target of adjuvant chemotherapy, in cases of metastatic cardiac tumor, was primary origin cancer, whether the tumor remained in the primary site at the time of surgery or not, variety of tumor specific protocols have been used.
The mean survival of all patients was 22.2 ± 6.1 months (range 0.6-36 months) and 1- and 2-yr survivals were 82.5% and 41.3%, respectively (Fig. 2). When the patients were divided into two groups, according to the completeness of tumor resection, survival of the incomplete resection group, except biopsy cases was 25.9 ± 7.9 months, and there was no mortality in the complete resection group. On the other hand, the mean survival of 32.3 ± 3.0 months in both surgical resection groups seemed to be better than that of 8.6 ± 8.1 months in 2 patients received biopsy although there was no statistical significance (P = 0.075). The mean survival of patients who had primary cardiac malignancies was 22.8 ± 6.3 months, but survival in cases of metastatic tumor showed 13.1 ± 2.3 months because almost all patients underwent the operation in the past few years and had shorter periods of follow up duration after surgery. There was no mortality in all patients (n = 7) who received adjuvant chemotherapy and/or radiation therapy. It might implicate the positive clinical impact on surgical outcomes and usefulness of adjuvant therapy because the mean survival of 4 patients without adjuvant therapy was 13.3 ± 3.9 months.
Malignant tumors of the heart and great vessels are very rare, but various types of malignancies or malignant like tumors could be identified (1). The most frequent primary malignant tumors are sarcomas, which may arise in any structures of the heart and great vessels, including pulmonary artery, aorta and vena cavae (14, 15). In general, the right atrial lesions are more frequently malignant, but the left sided atrial lesions are usually benign tumors (16). In the previous report, 75% of angiosarcomas, which are the most common cardiac sarcoma may occur in the right heart, especially in the right atrium (1). However, in our 4 cases, 2 cardiac sarcomas, including leiomyosarcoma and angiosarcoma, arose in the left ventricle and left atrium, and one case of undifferentiated sarcoma involved both atrium and interatrial septum. Only one angiosarcoma occurred in the right atrium and invaded the superior vena cava. Metastases to or direct invasion of the heart and great vessels are far more common than primary malignancies, and the incidence is known to be about 10%, based on autopsy cases in which malignant neoplasm was diagnosed. The most common tumor type is lung cancer, and other tumors, such as colon cancer, hepatocellular carcinoma, breast cancer, lymphoma, and melanoma have also been reported to metastasize to the heart and great vessels.
The clinical diagnosis is usually delayed because the malignancies are often asymptomatic until an advanced stage, and the manifestations are variable and nonspecific. It has been known that the classic triad of symptoms and signs result from intracardiac obstruction, systemic embolization and constitutional cause (17). The direct involvement of valves or significant obstruction of cardiac chambers, which interrupts blood flow, often causes congestive heart failure or arrhythmias. Therefore, the typical symptoms are progressive dyspnea, chest discomfort, palpitation, and edema. Almost all patients in our cohort complained with the symptoms above. However, 3 patients were asymptomatic and 2 cases of metastatic tumor were incidentally detected on a follow up CT angiogram at the outpatient clinics. One patient complained of left shoulder pain and hoarseness due to the tumor involvement of superior sulcus of the left lung, including clavicle, the first rib and left upper lobe. Moreover, two patients with adjacent lung cancer presented pulmonary symptoms, including cough, sputum or blood tinged sputum.
The preoperative diagnostic imaging modalities include echocardiography, CT angiogram, and cardiac MRI (18). Echocardiography is useful to identify the involvement and competence of intracardiac structures, including valves, ventricular function, and several determinants, which reflects the obstructive physiology of blood flow caused by the masses. CT angiogram is an important diagnostic tool for characterizing tumor masses, detecting metastasis to other organs, and determining the surgical respectability due to the high degree of tissue resolution (1). Cardiac MRI is recommended in cases of differentiating the tumors from myocardium, thrombus, vegetation, and artifact, as well as requiring more accurate assessment of tumor extent. Since the infiltrative growth and extracardiac extension of malignant cardiac tumors is better delineated by cardiac MRI (19), it may be more helpful to differentiate the malignant tumor from benign mass and to assess the resectability of the tumors than CT angiogram (Fig. 3). We have used the echocardiography and CT angiogram as the initial diagnostic imaging studies in all patients and often performed the cardiac MRI as an additive tool.
Surgical excision is mainstay treatment of primary or metastatic malignancies of the heart and great vessels because it can provide the symptom relief and hemodynamic improvement. Complete surgical resection was the only impact factor for the survival rate in the literature (7, 20-22). In patients with a limited tumor extent, a complete resection should be performed for improvement of survival, but the malignant cardiac tumors are usually large at the time of diagnosis, a complete resection is often difficult to achieve. Bakaeen et al. (9) mentioned that the single area that really renders resection impossible was involvement of the fibrous skeleton of the heart. They used several modifications on cardiopulmonary bypass and performed variable surgical techniques, including reconstruction of cardiac chambers and ex vivo tumor resection under cardiac explantation with reimplantation to achieve negative resection margin. We also tried to obtain the complete or debulking mass resection. Reconstruction of atrial chambers and great vessels with GA-fixed autologous pericardium or bovine pericardium, total arch replacement using the Spielvogel technique (13), arch vessel reconstruction with artificial T graft, concomitant valve surgery and additional pulmonary resection were performed in all patients. Even incomplete resection may provide substantial symptom-free survival (11). Two patients with malignant fibrous histiocytoma, which is an extremely rare tumor and usually occurs in the left atrium, underwent incomplete, but aggressive surgical resection, followed by adjuvant chemotherapy. Although one patient received reoperation for local recurrence, they are alive until now and shows 36 and 12 months of survival from first operation, respectively.
In metastatic cardiac tumors, although several cases of successful surgical resection have been reported, a surgical approach in these patients is usually limited to be palliative because in most cases these patients have limited life expectancies (1). In the present study, 3 of all patients with metastatic tumor, except one biopsy case and only great vessel involvement, underwent aggressive surgical resection of masses in the left atrium and pulmonary veins, followed by adjuvant chemotherapy. Of those, 2 cases that infiltrated to the right lung had combined bilobectomy of lung for the right middle lobe and lower lobe tumors. There was no evidence of disease status in these patients during the follow up period. Since they had the metastatic cardiac malignancies without residual cancer in the primary origin sites, such as the colon and lungs, the main purpose of surgery was not palliative but curative resection of mass, and therefore, there showed relatively good clinical outcomes.
Adjuvant chemotherapy and/or radiotherapy have been recommended for the tumor, and even complete surgical resection was obtained. However, the benefits of adjuvant therapy after surgery are yet unknown and several chemo- and radiotherapeutic regimens, as showed variable results (23). In our cases, there was no mortality in all patients (n = 7) who received adjuvant therapy while the mean survival of four patients without adjuvant therapy was 13.3 ± 3.9 months. Therefore, surgery, followed by adjuvant chemotherapy and/or radiation therapy may provide better palliation and improved survival.
Several limitations of this study need to be addressed. The small number of patients and descriptive analysis in the present study negates definite conclusions. Moreover, a risk factor analysis for postoperative survival and tumor recurrence was not performed because we focused on the clinical feature and surgical considerations. Further investigation may lead to a better understanding of surgical outcomes in malignancies of the heart and great vessels.
In conclusion, with aggressive and complete surgical resection for malignant tumor, involving the heart and great vessels, including adjacent tissue and organs suspicious of invasion, improved clinical outcomes could be obtained without life threatening complications. Although the part of malignacies cannot be resected completely with the curative intent, possible debulking for palliation also showed more reasonable results than only exploration and biopsy. Moreover, when the primary origin cancers are well controlled without residual tumors in patients with metastatic malignancies of heart and great vessels, more aggressive surgical resection for tumors should be considered because of better prognosis. Adjuvant multimodality therapy might be imperative to prevent local recurrence and distant metastasis, even in patients who underwent complete resection, and to provide improved survival.
Figures and Tables
Fig. 1
Intraoperative findings and surgical resection. (A) This picture shows the metastatic tumor originated from colon cancer which involved the LA and right lung. The tumor causes the obstruction of mitral valve (arrow). (B) En bloc tumor resection with intra-pericardial bilobectomy of right middle and lower lobe, LA plasty with glutaraldehyde (GA)-fixed autologous pericardial patch, mediastinal lymph node dissection. (C) Mediastinal mass involves the distal ascending aorta, total arch and arch vessels, superior vena cava, and innominate vein. Resection of superior vena cava (SVC) invasion (arrow). (D) Replacement of the ascending aorta and total arch with 18 mm vascular graft, and separate replacement of arch vessels using 12-8-8 mm Y-Yed graft (Spielvogel technique). SVC reconstruction with GA-fixed autologous pericardium (arrow).

Fig. 2
Kaplan-Meier survival curves for the overall survival rate for all patients with malignant tumor of the heart and great vessels.
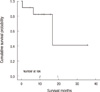
Fig. 3
Preoperative cardiac MRI and intraoperative findings of malignant cardiac tumor. (A) Well enhancing 4.8-cm size broad based mass in left atrium (LA) and interatrial septum extending to orifice of left lower pulmonary vein. (B) Tumor involvement to mitral valve anterior leaflet (arrow). (C) SVC transection with RA retraction, and LA roof approach for full exposure of mass: Broad based, 5×3 cm sized, multilobulating, large LA mass occupied the LA cavity including posterior and inferior wall, and invaded the mitral valve leaflet.
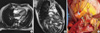
Table 2
Operative information

CPB, cardiopulmonary bypass; ACC, aorta cross clamp; LA, left atrium; MV, mitral valve; TV, tricuspid valve; SVC, superior vena cava; RA, right atrium; LAA, left atrial appendage; MVR, mitral valve replacement; MAP, mitral annuloplasty; RML, right milde lobe of lung; RLL, right lower lobe of lung; LUL, left upper lobe of lung; AV, atrioventricular.
References
1. Neragi-Miandoab S, Kim J, Vlahakes GJ. Malignant tumours of the heart: a review of tumour type, diagnosis and therapy. Clin Oncol (R Coll Radiol). 2007. 19:748–756.
2. Silverman NA. Primary cardiac tumor. Ann Surg. 1980. 191:127–138.
3. Dohi T, Ohmura H, Daida H, Amano A. Primary right atrial cardiac osteosarcoma with congestive heart failure. Eur J Cardiothorac Surg. 2009. 35:544–546.
4. Nayar S, Nayar PG, Cherian K. Angiosarcoma presenting as syncope. Asian Cardiovasc Thorac Ann. 2008. 16:154–156.
5. Yoshitake I, Hata M, Sezai A, Niino T, Unosawa S, Shimura K, Kasamaki Y, Minami K. Cardiac angiosarcoma with cardiac tamponade diagnosed as a ruptured aneurysm of the sinus valsalva. Jpn J Clin Oncol. 2009. 39:612–615.
6. Kitamura A, Ozaki N, Mukohara N, Yoshida M, Shida T. Primary cardiac liposarcoma causing cardiac tamponade: report of a case. Surg Today. 2007. 37:974–976.
7. Park BJ, Bacchetta M, Bains MS, Downey RJ, Flores R, Rusch VW, Girardi LN. Surgical management of thoracic malignancies invading the heart or great vessels. Ann Thorac Surg. 2004. 78:1024–1030.
8. Talbot SM, Taub RN, Keohan ML, Edwards N, Galantowicz ME, Schulman LL. Combined heart and lung transplantation for unresectable primary cardiac sarcoma. J Thorac Cardiovasc Surg. 2002. 124:1145–1148.
9. Bakaeen FG, Jaroszewski DE, Rice DC, Walsh GL, Vaporciyan AA, Swisher SS, Benjamin R, Blackmon S, Reardon MJ. Outcomes after surgical resection of cardiac sarcoma in the multimodality treatment era. J Thorac Cardiovasc Surg. 2009. 137:1454–1460.
10. Bear PA, Moodie DS. Malignant primary cardiac tumors: the Cleveland Clinic experience, 1956 to 1986. Chest. 1987. 92:860–862.
11. Mayer F, Aebert H, Rudert M, Königsrainer A, Horger M, Kanz L, Bamberg M, Ziemer G, Hartmann JT. Primary malignant sarcomas of the heart and great vessels in adult patients--a single-center experience. Oncologist. 2007. 12:1134–1142.
12. Zhang PJ, Brooks JS, Goldblum JR, Yoder B, Seethala R, Pawel B, Gorman JH, Gorman RC, Huang JH, Acker M, et al. Primary cardiac sarcomas: a clinicopathologic analysis of a series with follow-up information in 17 patients and emphasis on long-term survival. Hum Pathol. 2008. 39:1385–1395.
13. Spielvogel D, Strauch JT, Minanov OP, Lansman SL, Griepp RB. Aortic arch replacement using a trifurcated graft and selective cerebral antegrade perfusion. Ann Thorac Surg. 2002. 74:S1810–S1814.
14. Blackmon SH, Rice DC, Correa AM, Mehran R, Putnam JB, Smythe WR, Walkes JC, Walsh GL, Moran C, Singh H, et al. Management of primary pulmonary artery sarcomas. Ann Thorac Surg. 2009. 87:977–984.
15. Shanmugam G. Primary cardiac sarcoma. Eur J Cardiothorac Surg. 2006. 29:925–932.
16. Kubota H, Takamoto S, Kotsuka Y, Miyairi T, Murakawa T, Makuuchi H, Kawauchi M, Furuse A, Sudo K. Surgical treatment of malignant tumors of the right heart. Jpn Heart J. 2002. 43:263–271.
17. Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumors: diagnosis and management. Lancet Oncol. 2005. 6:219–228.
18. Debourdeau P, Gligorov J, Teixeira L, Aletti M, Zammit C. Malignant cardiac tumors. Bull Cancer. 2004. 91:136–146.
19. Kim CH, Dancer JY, Coffey D, Zhai QJ, Reardon M, Ayala AG, Ro JY. Clinicopathologic study of 24 patients with primary cardiac sarcomas: a 10-year single institution experience. Hum Pathol. 2008. 39:933–938.
20. Eckstein R, Gössner W, Rienmüller R. Primary malignant fibrous histiocytoma of the left atrium: surgical and chemotherapeutic management. Br Heart J. 1984. 52:354–357.
21. Burke A, Virmani R. Tumors of the heart and great vessels. Atlas of tumor pathology. 3rd series. 1996. Washington, D.C.: Armed Forces Institute of Pathology.
22. Kim MP, Correa AM, Blackmon S, Quiroga-Garza G, Weilbaecher D, Bruckner B, Ramlawi B, Rice DC, Vaporciyan AA, Reardon MJ. Outcomes after right-side heart sarcoma resection. Ann Thorac Surg. 2011. 91:770–776.
23. Putnam JB Jr, Sweeney MS, Colon R, Lanza LA, Frazier OH, Cooley DA. Primary cardiac sarcomas. Ann Thorac Surg. 1991. 51:906–910.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download