Abstract
Thyroid carcinomas are uncommon in childhood and adolescence. The aim of this study was to analyze clinical features and clinical outcomes of thyroid cancer in the pediatric population treated in the Yonsei University Health System. From September 1982 to June 2009, 90 patients (75 females, 15 males; female:male ratio of 5:1) with differentiated thyroid carcinoma were identified in our institute. The mean age at diagnosis was 15.8 yr old (range 4.8-19.9 yr). Cervical masses were most common clinical manifestations at diagnosis in 65 patients (72.2%). Forty-two patients underwent less than total thyroidectomy and 18 patients underwent total thyroidectomy. Thirty patients (33.3%) had lateral neck lymph node metastasis and seven patients (7.8%) had lung metastasis at the time of surgery. Among the 90 patients, recurrence occurred in 14 patients (15.5%). Mean follow-up period for patients with differentiated thyroid carcinoma was 81.6 months (13-324 months). No patients died of differentiated thyroid carcinoma. Patients with differentiated thyroid carcinoma who were < 20-yr-of-age were present with aggressive local disease and a high frequency of lymph node and distant metastasis. It is recommended that pediatric thyroid cancer should be managed mostly using proper surgical approach with thyroidectomy and lymph node dissection when indicated.
Primary thyroid carcinoma is uncommon in childhood and adolescence constituting 0%-3% of all pediatric malignancies (1-4). Sporadic differentiated thyroid cancers (papillary and mixed papillary-follicular) are the most common endocrine malignancies in children, and a female preponderance is most pronounced in postpubertal children (5). Follicular thyroid carcinoma is less common than papillary thyroid carcinoma in pediatric patients. Also, anaplastic and undifferentiated thyroid carcinomas are extremely rare. In the pediatric age group, sporadic medullary thyroid carcinoma (MTC) is uncommon and more frequently presents as a manifestation of multiple endocrine neoplasia (MEN) type IIA and IIB (6).
Differentiated thyroid carcinoma in pediatric patients differs from thyroid carcinoma in adults in clinical manifestations and clinical outcomes. Despite an overall survival rate exceeding 95%, pediatric thyroid carcinoma is often more advanced with lymph node metastasis and pulmonary metastasis at diagnosis and local recurrence is more frequent after thyroid operation than in adults (7-9). However, even though the stage is advanced, the prognosis is excellent for pediatric patients, with a low mortality rate.
Some studies have attempted to evaluate about the prognostic factor in pediatric thyroid cancer for the proper treatment but the prognostic factors have been evaluated poorly (9). Because of a lack of randomized studies, the optimal initial surgical treatment is controversial, and the impact of the initial surgery on the outcome remains unclear (3). Total thyroidectomy with lymph node dissection reduces the recurrence rate (10, 11), but carries a high risk of permanent hypoparathyroidism and vocal cord injury. Some investigators have advocated less radical surgery in pediatric patients (11, 12).
This study was analyzed the clinical features and clinical outcomes of thyroid cancer in the pediatric population at Yonsei University Health Service for 27 yr and discuss about the proper management of the differentiated thyroid cancer in pediatric population.
Between 1982 and 2009, 94 pediatric patients underwent thyroid surgery at Yonsei University Health System for primary thyroid carcinoma (differentiated thyroid carcinoma, 90 patients; medullary thyroid carcinoma, 4 patients; papillary thyroid carcinoma, 78 patients; follicular thyroid carcinoma, 12 patients). Patients with medullary thyroid carcinoma were excluded. Pediatric age is defined as < 20-yr. Retrospectively evaluated medical data was evaluated sex, age at the time of surgery, clinico-pathologic characteristics, TNM stage, type of surgery, postoperative complications, recurrences and survival rate. Clinico-pathologic characteristics included chief complaint at diagnosis, tumor size, extrathyroidal extension, multifocality, bilaterality, family history, thyroiditis and lymph node involvement. Pathologic staging adopted the 7th American Joint Committee on Cancer (AJCC) at the time of initial diagnosis.
At our institution, thyroid resection consisted of total thyroidectomy and less than total thyroidectomy. The definition of "less than total thyroidectomy" is specified by unilateral thyroid lobectomy, ipsilateral total and contralateral partial thyroidectomy and ipsilateral total and contralateral subtotal thyroidectomy. The choice of surgical extension was based on ATA guidelines (13). During thyroid resection, only central compartment lymph nodes (CCLN) located in the ipsilateral site of the primary tumor were dissected prophylatically. The CCLN or level VI included the pretracheal, paratracheal, prelaryngeal, perithyroidal nodes and lymph nodes located along the recurrent laryngeal nerve. The central compartment was delimited superiorly by the hyoid bone, inferiorly by the substernal notch, laterally by the median portion of the carotid sheath and dorsally by the prevertebral fascia. Recurrent laryngeal nerves and parathyroid glands were identified and preserved in all cases. The procedures were performed by the same surgical team. If patients had evidence of lateral lymph node metastasis in preoperative evaluation, modified radical neck dissection was performed. Radioactive iodine remnant ablation was performed within 6 weeks after surgery. Indication for radioiodine ablation in this cohort of patients was based on ATA guidelines (13). A 131I whole body scan was taken on day 2 following radioactive iodine (RI) therapy. Dose of radioactive iodine for children was calculated based on the patient's body weight (0.5-1.5 mCi/kg). All patients received thyroid-stimulating hormone suppression treatment with levothyroxine according to ATA guidelines (13). The recurrence after surgery was assessed by regular follow-up every 3 or 6 months. All patients in whom recurrence was suspected were confirmed using cytology and/or histology. Follow-up data was obtained by medical chart retrospectively until December 2011. This retrospective study was approved by the Institutional Review Board of Yonsei University Severance Hospital.
Ninety patients (75 females, 15 males; female:male ration, 5:1; 78 patients with papillary carcinoma, 12 patients with follicular carcinoma) underwent surgery for differentiated thyroid carcinoma in our institute. The mean age at diagnosis was 15.8 yr (females, 16.3 yr; males, 13.3 yr; range, 4.3-19.9 yr).
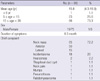
A neck mass was the most common chief complaint. Sixty five patients (72.2%) had a neck mass: in 50 patients, the mass was an anterior neck mass and in 15 patients it was a lateral neck mass. Other initial causes were incidentalomas in 18 cases (including patients who were followed-up due to thyroid disease and screening test), hoarseness in two cases and neck pain in one case. One patient underwent Sistrunk operation due to thyroglossal duct cyst, and the final diagnosis was confirmed as papillary thyroid carcinoma. One patient was diagnosed as thyroid carcinoma with lung metastasis during mumps treatment. In one patient, the lung metastasis was discovered during the pneumothorax operation and a wedge resection of the lung was performed, with papillary thyroid carcinoma subsequently confirmed. One patient had a previous history of exposure to ionizing radiation because of rhabdomyosarcoma in the left mandible. That patient had multiple malignancies which were chondrosarcoma in the left clavicle and osteosarcoma in the left knee. The patient treated chemotherapy and radiation therapy (Table 1). At the time of surgery, 60 patients had localized disease in central neck and 30 patients had lateral neck node metastasis. Seven patients had pulmonary metastasis at the time of surgery and all patients with pulmonary metastasis presented lateral neck node metastasis at the time of surgery.
Surgical treatment consisted of less than total thyroidectomy (42 patients, 46.7%), total thyroidectomy (18 patients, 20%) and total thyroidectomy with modified radical neck node dissection (30 patients, 33.3%). Thirty patients had lateral neck node metastasis, 16 patients underwent total thyroidectomy with central compartment node dissection (CCND) and unilateral modified radical neck dissection (MRND) and 14 patients underwent total thyroidectomy with CCND and bilateral MRND (Table 2).
Mean tumor size was 23.6 mm (range 2-60 mm). Microcarcinoma was present in 18 cases (20%) and six patients (6/18, 33.3%) had lateral neck node metastasis with microcarcinoma. Seventeen patients (18.9%) had bilaterality and 26 patients (28.9%) had multifocality. Extrathyroidal extension was evident in 51 cases (56.7%). Sixteen patients had diffuse thyroiditis (17.8%). Fifty four patients (60%) displayed central lymph node metastasis and 30 patients (33.3%) displayed lateral neck node metastasis. In the 30 patients without central lymph node metastasis, three patients (10%) displayed lateral neck node metastasis. Two patients had recurrent laryngeal nerve invasion, during the operation the nerve was dissected for the treatment (Table 3). In addition, concerning the TNM stage, stage I and stage II comprised 83 cases (92.2%) and seven cases (7.8%), respectively.
Postoperative RI was administered to 41 of 48 patients (85.4%) presenting with lung metastasis at initial diagnosis. Mean age of the 41 patients (nine males, 32 females, 1:3.6 ratio) was 15.6 yr (range 5-19.9 yr). The tumor size was 23.5 ± 12.6 mm. The RI dose ranged from 30 mCi to 750 mCi in initial non-pulmonary metastasis patients. In patients with initial pulmonary metastasis who received RI therapy, the dose ranged from 150-1,050 mCi (1-5 times). One patient received external beam radiation therapy.
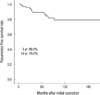
The mean follow-up duration was 81.6 months (range 13-324 months) and no patients died of thyroid carcinoma. During the follow-up period, recurrence occurred in 14 patients (15.6%). Recurrence was in the lateral neck (n = 6, 42.9%), operation bed (n = 4, 28.6%), contra-lateral thyroid gland and lateral neck (n = 2, 14.3%), remaining contra-lateral thyroid gland (n = 1, 7.1%) and operation bed and lateral neck (n = 1, 7.1%) (Table 4). These patients were treated with appropriate therapy, such as completion total thyroidectomy, MRND and RI treatment. The recurrence-free survival rate was 88.9% at 5 yr and 78.9% at 10 yr from initial operation (Fig. 1). Two patients died with a nonspecific disease cause. One patient died with intracranial hemorrhage due to leukemia. The other patient with disseminated rhbdomyosarcoma died with multi-organ failure. No patients died of differentiated thyroid carcinoma. After the initial operation, five of the 14 patients with recurrence had lung metastasis and were treated with RI and thyroid stimulating hormone (TSH) suppression.
Eighteen patients (20%) had a cancer with a tumor size ≤ 10 mm. Mean age was 16.9 yr (range, 6-19 yr) and the male:female ratio was 1:17. Nine patients were diagnosed incidentally in the follow-up period with thyroid disease (goiter, hypothyroidism and hyperthyroidism) and screening test, six patients had an anterior neck mass and three patients had a lateral neck mass. Mean tumor size was 6.4 ± 2.6 mm (range, 3-10 mm). One patient had bilaterality and four patients had multifocality. Extrathyroidal extension occurred in eight cases. Six patients had diffuse thyroiditis. Central lymph node metastasis was evident in 12 of 18 patients (66.7%). Six of 18 patients (33.3%) with lateral neck node metastasis received total thyroidectomy with central lymph node dissection and ipsilateral modified radical neck dissection. After the initial surgery, only one patient experienced recurrent thyroid cancer in the operation bed.
At the time of surgery, lung metastasis was detected in seven cases (7.8%) of papillary thyroid carcinoma. After surgical excision (total thyroidectomy with CCND with/without MRND), radioactive iodine and TSH suppression therapy were added, considering the body weight of patient. External radiation therapy was administered to one of seven patients with pulmonary metastasis.
Twenty four patients (26.7%) had postoperative complication with hypocalcemia (transient in 19 patients and permanent in 5 patients). No incidental recurrent laryngeal nerve injury was noted, but two cases were resected intentionally due to the encasement of the tumor to the recurrent laryngeal nerve. Postoperative bleeding and infection were not noted.
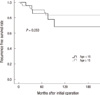
After grouping patients with thyroid carcinoma into two groups according to the age at diagnosis (children, age < 15 yr; adolescents, 15 ≤ age ≤ 20 yr), their clinical and pathological characteristics were compared (Table 5). When the clinicopathologic characteristics of patients with thyroid carcinoma were compared according to age group, there were no statistically significant differences among the age groups in terms of pathologic diagnosis proportion of papillary thyroid carcinoma, presence of thyroiditis, central lymph node metastasis rate and distant metastasis rate. However, there were significant differences between the two groups in terms of gender ratio, tumor size, operation method, extrathyroidal extension, bilaterality, multifocality and lateral lymph node metastasis rate. The male:female ratio was 1:1.18 and 1:15.5 in the < 15-yr-old group and ≥ 15-yr-old group, respectively. Although both groups had a higher proportion of females, the proportion of males was significantly higher in the younger age group (P= 0.001). As well, this younger age group had more aggressive factors such as high extrathyroidal extension, bilaterality, multifocality and larger tumor size(P = 0.001, P = 0.001, P = 0.016, and P = 0.007, respectively). Although those < 15-yr-old group had more aggressive factors, there were no significant differences between two groups in terms of the recurrence free survival rate (P = 0.258, Fig. 2) and recurrence rate (P = 0.187).
Thyroid carcinoma in the pediatric population is a rare type of malignancy that displays different tumor characteristics compared with adult thyroid cancers. Pediatric thyroid cancer is aggressive, has a higher prevalence of lymph node metastasis and pulmonary metastasis at the time of initial diagnosis and occurs more frequently after surgery (7-9, 14). However, the prognosis is excellent. The reason for this discrepancy is unclear, but some hypotheses for pediatric thyroid cancer have been suggested. Zimmerman et al. reported that nondiploid DNA amount was 10% in pediatric thyroid cancer and 20% in adult thyroid cancer. The authors suggested that a low incidence of nondiploid DNA was related to the good prognosis in pediatric thyroid cancer (8). The other possibility is that the thyroid gland during infancy and childhood is more susceptible to carcinogenic stimuli. TSH may play a more prominent role as a promoting factor in younger patients. Therefore, postoperative TSH suppression with thyroid hormone replacement is more effective and the dedifferentiation from well differentiated to poorly differentiated carcinoma does not often occur in pediatric thyroid carcinoma (9, 15).
Some molecular-based studies were performed. But, the biologic basis for such a difference is essentially unknown. Several studies have reported that BRAF V600E mutations are more common in older patients with aggressive papillary thyroid cancer (16), and that RET/PTC1 rearrangement are more common in children with papillary thyroid cancer (17, 18).
In our study, the incidence of thyroid carcinoma increased with age ( < 5 yr, n = 1; 5-15 yr, n = 23; 15-20 yr, n = 66), with the oldest age group comprising 73.3% of the total patients. Our study shows high incidence of lymph node metastasis (60%). The incidence of lymph node metastasis in the pediatric group was similar to adults, in whom the prevalence of the subclinical central lymph node metastasis has been reported up to 80% in adult thyroid cancer (19, 20).
Fourteen patients (15.6% of total) recurred in our study. This rate of recurrence was substantially lower previous values of 35-40% (1, 4, 7, 8). Among the cases of differentiated pediatric thyroid cancer, 12 patients with follicular thyroid cancer did not recur. In our study, the incidence of central lymph node metastasis was high but the recurrence rate was low. This low incidence of recurrence rate supports the use of our institutional policy - that prophylactic central lymph node dissection is needed in thyroid cancer patients even if the tumor size is small - for both pediatric and adult patients.
Concerning the relationship of multiple factors, which some studies argued predictive factors for recurrence in pediatric thyroid cancer, our study did not reveal any relationship between recurrence and factors such as age, gender, extracapsular invasion, bilaterality, multifocality, thyroiditis, central lymph node metastasis, lateral neck node metastasis, type of operation and RI therapy. The possible relationship between patient age and recurrence has been studied. Some studies reported higher recurrence rate for children < 10 yr or < 15 yr (4, 7), but others did not confirmed this relationship (21).
The most impressive finding in the present study is that 18 patients (18/90, 20%) had papillary thyroid microcarcinoma (PTMC). PTMC patients comprise 3.1%-18.2% of those with lymph node metastasis and up to 20% of locoregional recurrences, although PTMC is usually indolent and curable with surgical thyroidectomy often followed by RI therapy in adult thyroid cancer (22-24). However, this study showed a higher incidence of central lymph node metastasis (12/18, 66.7%) and lateral neck node metastasis (6/18, 33.3%). On the other hand, the recurrence rate of PTMC patients was low (1/18, 5.6%) after initial surgery. PTMC patients did not have lung metastasis and recurrence even though the patients had lateral neck node metastasis. Among six patients with lateral neck node metastasis, one case had skip metastasis, with in no central lymph node metastasis with lateral neck node metastasis only. Even though the primary cancer size was < 1 cm, the chief complaint was palpable mass in the neck (5/6, 83.3%) due to the metastatic lymph node in the PTMC patients with lateral neck node metastasis. Until now, the studies about pediatric PTMC have been rare. Even the present findings are limited by small number of patients and short follow-up period. The present data is insufficient to make any conclusion in pediatric PTMC. However we can conclude that PTMC in pediatric thyroid cancer is not rare and has a high incidence of lymph node metastasis, low incidence of recurrence and that pediatric PTMC is possible metastasis to the lateral neck like adult.
The treatment of the pediatric thyroid cancer remains contentious. Because of the low incidence of pediatric thyroid cancer, a prospective trial is difficult to perform. Some studies supported the idea that a total thyroidectomy with central lymph node dissection by experts is safe and does not increase the possibility of serious complications (25). On the other hand, a conservative stepwise approach has been recommended to prevent surgical complications and to avoid over-surgery for non-advanced disease in pediatric thyroid cancer patients (8). Our results suggest that, even though the prognosis is excellent in pediatric thyroid cancer, proper surgery including lymph node dissection is needed to reduce the recurrence after surgery. Of course, TSH suppression treatment is required. 131I dose administered is adjusted by weight and by additional safety factors dependent on age if it is indicated.
In conclusion, our study shows that pediatric thyroid cancer has aggressive features at initial diagnosis but that the recurrence rate and mortality rate are low. The most common reason for hospital care in pediatric patients with papillary thyroid carcinoma was a palpable neck mass. In a pediatric population, a neck mass should be considered an indication of thyroid cancer. If pediatric thyroid cancer is diagnosed, further work-up should be performed due to the possibility of lateral neck node metastasis and distant metastasis. Pediatric thyroid cancer can be managed mainly using the proper surgical approach with total thyroidectomy and lymph node dissection when indicated.
Figures and Tables
References
1. Millman B, Pellitteri PK. Thyroid carcinoma in children and adolescents. Arch Otolaryngol Head Neck Surg. 1995. 121:1261–1264.
2. Thompson GB, Hay ID. Current strategies for surgical management and adjuvant treatment of childhood papillary thyroid carcinoma. World J Surg. 2004. 28:1187–1198.
3. Haveman JW, van Tol KM, Rouwé CW, Piers DA, Plukker JT. Surgical experience in children with differentiated thyroid carcinoma. Ann Surg Oncol. 2003. 10:15–20.
4. Borson-Chazot F, Causeret S, Lifante JC, Augros M, Berger N, Peix JL. Predictive factors for recurrence from a series of 74 children and adolescents with differentiated thyroid cancer. World J Surg. 2004. 28:1088–1092.
5. Howe HL, Wu X, Ries LA, Cokkinides V, Ahmed F, Jemal A, Miller B, Williams M, Ward E, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975-2003, featuring cancer Hispanic/Latino populations. Cancer. 2006. 107:1711–1742.
6. Chadha NK, Forte V. Pediatric head and neck malignancies. Curr Opin Otolaryngol Head Neck Surg. 2009. 17:471–476.
7. La Quaglia MP, Corbally MT, Heller G, Exelby PR, Brennan MF. Recurrence and morbidity in differentiated thyroid carcinoma in children. Surgery. 1988. 104:1149–1156.
8. Zimmerman D, Hay ID, Gough IR, Goellner JR, Ryan JJ, Grant CS, Mc-Conahey WM. Papillary thyroid carcinoma in children and adults: longterm follow-up of 1039 patients conservatively treated at one institution during three decades. Surgery. 1988. 104:1157–1166.
9. Koo JS, Hong S, Park CS. Diffuse sclerosing variant is a major subtype of papillary thyroid carcinoma in the young. Thyroid. 2009. 19:1225–1231.
10. Welch Dinauer CA, Tuttle RM, Robie DK, McClellan DR, Francis GL. Extensive surgery improves recurrence-free survival for children and young patients with class I papillary thyroid carcinoma. J Pediatr Surg. 1999. 34:1799–1804.
11. Stael AP, Plukker JT, Piers DA, Rouwé CW, Vermey A. Total thyroidectomy in the treatment of thyroid carcinoma in childhood. Br J Surg. 1995. 82:1083–1085.
12. Steinmüller T, Klupp J, Wenking S, Neuhaus P. Complications associated with different surgical approaches to differentiated thyroid carcinoma. Langenbecks Arch Surg. 1999. 384:50–53.
13. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009. 19:1167–1214.
14. Viswanathan K, Gierlowski TC, Schneider AB. Childhood thyroid cancer: characteristics and long-term outcome in children irradiated for benign conditions of the head and neck. Arch Pediatr Adolesc Med. 1994. 148:260–265.
15. Buckwalter JA, Thomas CG, Freeman JB. Is childhood thyroid cancer a lethal disease? Ann Surg. 1975. 181:632–639.
16. Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005. 12:245–262.
17. Nikiforov YE. RET/PTC rearrangement in thyroid tumors. Endocr Pathol. 2002. 13:3–16.
18. Nikiforov YE, Rowland JM, Bove KE, Monforte-Munoz H, Fagin JA. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 1997. 57:1690–1694.
19. Noguchi S, Noguchi A, Murakami N. Papillary carcinoma of the thyroid: I. developing pattern of metastasis. Cancer. 1970. 26:1053–1060.
20. Attie JN, Khafif RA, Steckler RM. Elective neck dissection in papillary carcinoma of the thyroid. Am J Surg. 1971. 122:464–471.
21. Handkiewicz-Junak D, Wloch J, Roskosz J, Krajewska J, Kropinska A, Pomorski L, Kukulska A, Prokurat A, Wygoda Z, Jarzab B. Total thyroidectomy and adjuvant radioiodine treatment independently decrease locoregional recurrence risk in childhood and adolescent differentiated thyroid cancer. J Nucl Med. 2007. 48:879–888.
22. Bramley MD, Harrison BJ. Papillary microcarcinoma of the thyroid gland. Br J Surg. 1996. 83:1674–1683.
23. Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid: a "normal" finding in Finland: a systematic autopsy study. Cancer. 1985. 56:531–538.
24. Pelizzo MR, Boschin IM, Toniato A, Pagetta C, Piotto A, Bernante P, Casara D, Pennelli G, Rubello D. Natural history, diagnosis, treatment and outcome of papillary thyroid microcarcinoma (PTMC): a mono-institutional 12-year experience. Nucl Med Commun. 2004. 25:547–552.
25. Hallwirth U, Flores J, Kaserer K, Niederle B. Differentiated thyroid cancer in children and adolescents: the importance of adequate surgery and review of literature. Eur J Pediatr Surg. 1999. 9:359–363.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download