Abstract
Microscopic anthracotic pigment (MAP) is frequently observed in endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) specimen in non-small cell lung cancer, but its clinical interpretation is not well-known. The aim of this study was to evaluate the clinical implication of MAP in mediastinal staging of non-small cell lung cancer. From May 2010 to July 2011, consecutive potentially operable non-small cell lung cancer patients who underwent EBUS-TBNA for mediastinal staging were recruited. Of the total 133 patients, 102 (76.7%) were male patients. Median age was 68 yr. Total 279 mediastinal lymph nodes were sampled by EBUS-TBNA; station 4R (100, 35.8%) and station 7 (86, 30.8%) were the most common sites. Malignant lymph nodes were 100 (35.8%). MAP was observed in 61 (21.7%) lymph nodes, and among them only 3 were malignant lymph nodes (P < 0.001). The lymph nodes with MAP were smaller (9.0 vs 10.8 mm, P = 0.001) and showed low standard uptake values on FDG-PET (4.4 vs 4.7, P = 0.256). In multivariate analysis, MAP was negatively associated with malignant lymph node (adjusted OR, 0.12; 95% CI, 0.03-0.42; P < 0.001). In potentially operable non-small cell lung cancer patients, MAP in endobronchial ultrasound-guided transbronchial needle aspiration specimens is strongly associated with benign mediastinal and hilar lymph nodes.
The introduction of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) was a great advance in diagnostic bronchoscopy, especially in mediastinal staging of non-small cell lung cancer (1-5). From the aspirate and tissue cores of lymph nodes obtained by EBUS-TBNA, cytopathologic examination can be performed in the clinically suspected mediastinal and hilar lymph nodes. Because of its high diagnostic yields and minimally invasive nature, EBUS-TBNA is considered as an alternative method to mediastinoscopy (6).
According to the guidelines of invasive mediastinal staging of lung cancer (7), discrete lymph nodes on chest computed tomography (CT) or those with high F-18 fluorodeoxyglucose (FDG) uptake on positron emission tomography (PET) should be evaluated. Usually those lymph nodes are evaluated by EBUS. In some cases, although lymph nodes showed vivid FDG uptake, cytopathologic results revealed no evidence of malignancy, in other words false positive in PET. The most common causes are tuberculosis (8) and anthracosis (9).
Anthracosis is a kind of pneumoconiosis, and the term of "anthracotic" is related with black pigment, mostly due to carbon particle. Usually the deposition of black pigment is located in the macrophages of the mucosa and submucosa. Anthracosis is associated with active or old tuberculosis infection, smoking, air pollution, and biomass (10, 11). Anthracosis itself is benign disease, but anthracosis can confuse clinicians by appearing as false positive PET in mediastinal staging of lung cancer. Unfortunately the clinical implication of anthracotic pigmentation in mediastinal lymph node of lung cancer is unknown. The aim of this study was to evaluate the clinical interpretation of the presence of anthracotic pigment in EBUS-TBNA.
Patients who were admitted for lung cancer evaluation to Seoul National University Hospital from May 2010 to July 2011 were recruited. Among them, consecutive patients who underwent EBUS-TBNA for mediastinal staging in potentially operable non-small cell lung cancer were included in this retrospective study. We excluded the patients who had distant metastasis and inoperable T4 disease based on the 7th TNM staging (12).
All EBUS-TBNA was performed using a real-time linear probe (BF-UC260F-OL8; Olympus, Tokyo, Japan). We used 22-guage needle (NA-201SX-4022; Olympus) for transbronchial aspiration. The procedures were performed under local anesthesia (lidocaine) to the tracheobronchial tree and conscious sedation (midazolam). After detection of the target lymph nodes, puncture was done based on bronchoscopists' decision. The nodal station was classified according to the 7th TNM classification (13).
The tissue preparation of EBUS-TBNA has been described previously (3). In brief, the entire aspirate was expelled on glass slide, then we identified tissue core immediately and the tissue core was fixed in 10% formalin for the staining with hematoxylin and eosin (HE). The remaining aspirate was smeared and fixed with 95% alcohol for the staining with Diff Quick and Papanicolaou. Finally, remnants of aspirate were flushed with saline and collected for cell block. Rapid on-site evaluation (ROSE) was not available in our institution.
The malignant lymph node was defined as pathologic confirmation of malignant cells in EBUS-TBNA or surgical biopsy. And the benign lymph node was confirmed after surgical lung resection and mediastinal lymph nodes dissection, even though the results of EBUS-TBNA were benign.
The presence of MAP was evaluated by the pathologists under the microscopic examination of aspiration and histologic specimen. Fig. 1 shows the representative pictures of MAP from EBUS-TBNA specimens.
P values were calculated using the Chi-square test or Fisher's exact test for categorical variables and the Mann-Whitney U-test for continuous variables. Odds ratios (ORs) were calculated from the binary logistic regression model in the multivariate analysis. We considered P value < 0.05 as statistically significant. SPSS 19.0 (Chicago, IL, USA) was used for the analysis.
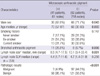
Among the total of 133 potentially operable non-small cell lung cancer patients, 102 (76.7%) were male (Table 1). The median age was 68 yr. Ninety seven patients (72.9%) had smoking history. During white light bronchoscopy, bronchial anthracotic pigment was observed in 19 patients (14.3%) and none of 133 patients had any evidence of anthracofibrosis. The most common pathologic diagnosis was squamous cell carcinoma (44.4%) followed by adenocarcinoma (42.9%). Most of the patients were clinical stage IIIA (36.1%) at the time of diagnosis.
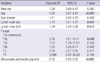
Among 133 patients, 279 hilar and mediastinal lymph nodes were punctured (Table 2). Median short diameter on chest CT was 9.8mm and median standard uptake value (SUV) on PET was 5.8. The most common punctured nodal station was station 4R (35.8%) followed by station 7 (30.8%). Among 279 lymph nodes, 100 (35.8%) were pathologically malignant. MAP was observed in 61 (21.9%) lymph nodes.
We calculated the diagnostic yield of EBUS-TBNA using these 133 patients and 279 lymph nodes. The sensitivity, specificity, positive predicted value (PPV), negative predicted value (NPV), and diagnostic accuracy were 94.0%, 98.2%, 98.4%, 93.2%, and 95.9%.
We compared the clinical characteristics according to the presence of MAP (Table 3). Male (P = 0.043) and old age (P = 0.003) was associated with MAP but smoking history was not. Hilar and mediastinal lymph node with MAP were smaller than the lymph node without MAP (8.5 vs 10.1 mm, P = 0.001) but SUVs was not different (P = 0.256). In 61 lymph nodes with MAP, only 3 (4.9%) showed malignant result (P < 0.001). In multivariate analysis using binary logistic regression, MAP was strongly associated with benign pathologic results (adjusted OR, 0.12, 95% confidence interval, 0.003-0.42, P < 0.001) (Table 4).
This present study showed the clinical implication of MAP in mediastinal staging of non-small cell lung cancer. To the best of our knowledge, this is the first mention of anthracotic pigment in EBUS-TBNA specimen. The lymph nodes with MAP were metabolically active, which showed high FDG uptake, but usually were histologically benign. The false positive PET results could confuse the clinicians. As we mentioned above, the lymph nodes with MPA were small (median 8.5 mm) but showed high SUV (median 4.4) in our study population. And only 3 (4.9%) were malignant lymph nodes.
Bronchial anthracotic pigmentation is a common finding of white light bronchoscopic examination. However, mediastinal lymphadenopathy caused by anthracosis was sparse in the literature review. Only a few cases were reported as a false positive FDG PET finding (9). Through our results, we could estimate the prevalence of bronchial anthracotic pigmentation and MAP in non-small cell lung cancer patients. Among 133 patients, 19 patients (14.3%) had bronchial anthracotic pigmentation and 41 patients (30.8%) had MAP in their hilar and mediastinal lymph nodes. The relatively high prevalence of bronchial anthracotic pigmentation and MAP could possibly be associated with tuberculosis (14). Several decades ago, Korea had a high burden of tuberculosis (15).
According to our results, hilar and mediastinal lymph nodes with MAP which showed small size but high SUV uptake, have significantly higher probability of being benign lymph nodes in non-small cell lung cancer. A possible explanation is false positive FDG-PET. The lymph nodes with MAP usually showed high SUV on PET, so bronchoscopists puncture those suspected lymph nodes. In other words, the bronchoscopists usually punctured that lymph node not because of discrete size but high FDG uptake. And another possible mechanism is that the accumulation of anthracotic particles could impair trapping malignant cells in lymph nodes. Murakami and Taniguchi (16) observed impaired nodal function and shunt flow in lymph nodes with anthracosis.
There are some limitations in this study. As we collected these data retrospectively, we could not assess the macroscopic feature of aspirates with naked eye. So we could not evaluate the correlation between macroscopic and microscopic features. In a clinical setting where ROSE is not available, macroscopic feature of aspirate may be more important for the bronchoscopists. The second, the prevalence of anthracosis is varied in geographic and ethnic difference. So, further studies are needed for generalization. And the third, the possibility of false negative result in EBUS-TBNA. To clarify the accuracy of EBUS-TBNA in our data, we calculated the false negative rate as 6.0%. The authors considered that as an acceptable range for analysis.
In conclusion, MAP in EBUS-TBNA specimen is strongly associated with benign lymph nodes in potentially operable non-small cell lung cancer patients.
Figures and Tables
 | Fig. 1Microscopic anthracotic pigment in lymph node acquired from endobronchial ultrasound-guided transbronchial needle aspiration. Dark brown pigment was observed in the inflammatory cells in (A) aspiration cytology (staining with Papanicolaou) and (B) tissue core (staining with hematoxylin and eosin) from the same patient (×400). |
References
1. Yasufuku K, Chiyo M, Sekine Y, Chhajed PN, Shibuya K, Iizasa T, Fujisawa T. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest. 2004. 126:122–128.
2. Herth FJ, Krasnik M, Kahn N, Eberhardt R, Ernst A. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest. 2010. 138:790–794.
3. Hwangbo B, Lee GK, Lee HS, Lim KY, Lee SH, Kim HY, Lee HS, Kim MS, Lee JM, Nam BH, et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest. 2010. 138:795–802.
4. Haas AR, Vachani A, Sterman DH. Advances in diagnostic bronchoscopy. Am J Respir Crit Care Med. 2010. 182:589–597.
5. Joo H, Kim HR, Oh YM, Kim YH, Shim TS, Kim DK, Park SI, Kim WS, Kim DS, Choi CM. The efficacy of endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer in a university hospital. Tuberc Respir Dis. 2011. 71:180–187.
6. National Comprehensive Cancer Network. NCCN guideline for patients: non-small cell lung cancer: National Comprehensive Cancer Network. accessed on 26 October 2011. Available at
http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
7. Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA. American College of Chest Physicians. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007. 132:202S–220S.
8. Li Y, Su M, Li F, Kuang A, Tian R. The value of 18F-FDG-PET/CT in the differential diagnosis of solitary pulmonary nodules in areas with a high incidence of tuberculosis. Ann Nucl Med. 2011. 25:804–811.
9. Cheng NM, Yeh TW, Ho KC, Ng SH, Hsueh C, Yen TC, Liao CT. False positive F-18 FDG PET/CT in neck and mediastinum lymph nodes due to anthracosis in a buccal cancer patient. Clin Nucl Med. 2011. 36:963–964.
10. Chung MP, Lee KS, Han J, Kim H, Rhee CH, Han YC, Kwon OJ. Bronchial stenosis due to anthracofibrosis. Chest. 1998. 113:344–350.
11. Gupta A, Shah A. Bronchial anthracofibrosis: an emerging pulmonary disease due to biomass fuel exposure. Int J Tuberc Lung Dis. 2011. 15:602–612.
12. Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007. 2:706–714.
13. Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P. Members of IASLC Staging Committee. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009. 4:568–577.
14. Hwang J, Puttagunta L, Green F, Shimanovsky A, Barrie J, Long R. Bronchial anthracofibrosis and tuberculosis in immigrants to Canada from the Indian subcontinent. Int J Tuberc Lung Dis. 2010. 14:231–237.
15. Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis. 1998. 2:27–36.
16. Murakami G, Taniguchi I. Histologic heterogeneity and intranodal shunt flow in lymph nodes from elderly subjects: a cadaveric study. Ann Surg Oncol. 2004. 11:279S–284S.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download