Abstract
Induction of apoptosis in target cells is a key mechanism by which chemotherapy promotes cell killing. The purpose of this study was to determine whether Indole-3-Carbinol (I3C) and Genistein in combination with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induce apoptosis in endometrial cancer cell (Ishikawa) and to assess apoptotic mechanism. The MTT assay and flow cytometry were performed to determine cell viability and cell cycle. The induction of apoptosis was measured by caspase-3 activity test, DNA fragmentation assay, annexin V binding assay and western blot analysis. There was no effect in cell growth inhibition and cell cycle progression alone or in two-combination. However, the treatment of I3C and Genistein followed by TRAIL showed significant cell death and marked increase in sub-G1 arrest. Three-combination treatment revealed elevated expression of DR4, DR5 and cleaved forms of caspase-3, caspase-8, PARP. The Flip was found down regulated. Moreover, increase in caspase-3 activity and DNA fragmentation indicated the induction of apoptosis. The results indicate that I3C and Genistein with TRAIL synergistically induced apoptosis via death receptor dependent pathway. Our findings might provide a new insight into the development of novel combination therapies against endometrial cancer.
Endometrial cancer is the fourth most common gynecologic malignancy in women in developed countries (1) which arises from the endometrial lining of the uterus. Patients with early stage disease have good prognosis and can be cured with surgery but there is no effective therapy for advanced or recurrent endometrial cancer (2). The incidence and mortality rates of endometrial carcinomas have increased in recent years. This emphasizes the need for a detailed understanding of the mechanism of the disease. From clinical perspectives, it is desirable to concomitantly target the molecular abnormalities by using a combination drug or agent therapy to optimize therapeutic outcomes (3). To improve the treatment strategy of the problem, we investigated the combination effect of Indole-3-Carbinol (I3C), Genistein and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) comparatively in nontoxic doses.
I3C, a common phytochemical in the human diet, is present in all members of the cruciferous vegetable family which includes cabbage, broccoli, brussels sprouts, cauliflower and kale. It has multiple facets of oncogenic signaling and is considered a promising agent for the protection of estrogen enhanced cancers (4). Genistein is a soy-derived isoflavone with multiple biochemical effects, including the cell cycle regulatory kinase activities (5, 6). It is believed to be a potent anticancer agent and found to inhibit tyrosine protein kinase (7). TRAIL is a member of the tumor necrosis factor gene superfamily that displays great apoptosis inducing activity against cancer cells both in vitro and in vivo (8) and known to bound death receptors via their intracellular death domain (9). TRAIL has been shown to be safe following administration in mice (10). However, many tumors remain resistance to the treatment with TRAIL and this resistance may be caused by the deregulated expression of apoptosis related molecules. Thus combination regimes need to be identified that potently enhances TRAIL mediated apoptosis.
The aim of our study was to further elucidate the synergistic interaction between I3C, Genistein and TRAIL for the treatment of endometrial cancer. The enhancement of TRAIL-mediated programmed cell death by phytochemicals may support antitumor immune responses. Therefore, we tested the cytotoxic and apoptotic effects of I3C and Genistein in combination with TRAIL in endometrial cancer. The combination treatment may offer a promising new approach in effective treatment of human endometrial cancers.
Dulbecco's modified eagle medium was obtained from GIBCO BRL (Grand Island, NY, USA). Indole-3-Carbinol, Genistein and TRAIL were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Enzo Life Sciences (Farmingdale, NY, USA), respectively. The human endometrial cancer cell line Ishikawa was obtained from European collection of cell cultures (ECACC, Salisbury, UK). The cells were cultured and incubated at 37℃ in 5% CO2.
The number of viable cell exposed to I3C, Genistein and TRAIL were evaluated by a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Initially, cells were seeded in 96-well plate, and then cultured for 24 hr to allow their adhesion to the plate. After pre-incubation, the culture medium was changed to experimental medium supplemented with DMSO (0.1%) control, I3C (50 µM), Genistein (20 µM) and TRAIL (10, 20, and 50 ng/mL) alone or in combination for 24 hr. The intensity of the purple color formed by this assay is proportional to the number of viable cells. MTT reagent was added and incubated for an additional 4 hr at 37℃. The optical density (OD) was measured at 540 nm. The mean values and their standard deviations were calculated from triplicate experiments.
To determine the distribution of cells in the different phases of cell cycle profile, flow cytometric analysis was performed. Cells were treated with DMSO (control) or I3C (50 µM), Genistein (20 µM) and TRAIL (10, 20, and 50 ng/mL) alone or in combination. After treatment, cells were harvested by trypsinization and centrifugation, washed with ice-cold phosphate buffered saline (PBS), and was fixed in ice-cold 70% ethanol at 4℃ for 24 hr. Ethanol fixed cells were washed and treated with RNase A for 30 min at 37℃ and were stained with propidium iodide. DNA fluorescence was measured by flow cytometer using a FACS Calibur cell sorter (Becton Dickinson, San Jose, CA, USA). The staining of apoptotic cells was performed by using annexin VFITC/PI apoptosis detection kit (BD Pharmingen, San Diego, CA, USA) according to the manufacturer's instructions. The determination of apoptotic cells was calculated using Cellquest software (Becton Dickinson).
Endometrial cancer cells were treated with DMSO (control) or I3C (50 µM), Genistein (20 µM) and TRAIL (10, 20, and 50 ng/mL) alone or in combination for 24 hr. After drug treatment, cells were harvested with lysis buffer. DTT Mix and 2 × Reaction buffer was added in supernatants and incubated on ice for 30 min. Caspase-3 substrate (DEVD-pNA; 50 µM final concentration) was added and incubated at 37℃ for 1 hr in water bath. Caspase-3 activity was measured by microplate reader (Tecan, Durham, NC, USA) at 405 nm followed by ApoAlert Caspase Colorimetric Assay kits User Manual (Clontech, Mountain View, CA, USA).
Cells were harvested, centrifuged and washed once in PBS. Cell pellets were lysed in DNA isolation buffer and centrifuged at 4℃ for 20 min. After treated with Tris saturated phenol (pH 8.0), the extraction was further treated with equal volume of phenol/chloroform/isoamyl alcohol (25:24:1 v/v) and chloroform/isoamyl alcohol (24:1 v/v) separately. The DNA was precipitated by addition 0.1 volume of 3 M sodium acetate and two volume of ice-cold ethanol and incubated at -70℃ for at least 2 hr. The samples were then loaded into a 1.8% agarose gel and electrophoresed for 40 min at constant 100V. DNA was visualized by UV illumination.
Fragmented nucleosomal DNA was quantified by Cell Death Detection ELISA Plus kit (Roche Diagnostics, Mannheim, Germany) as described in the manufacture's manual. The absorbance was measured at 405 nm using microplate reader (Tecan).
Cell (4 × 105/mL) extracts were prepared in lysis buffer (10 mM Tris [pH 7.4], 5 mM EDTA, 130 mM NaCl, 1% Triton X-100, serine protease inhibitor phenylmethylsulphonyl fluoride [10 µg/mL], leupeptin [10 µg/mL], aprotinin [10 µg/mL], 5 mM phenanthroline and 28 mM benzamidine-HCl). Protein concentrations were measured using Bio-Rad Protein Assay Reagent (Bio-Rad, Hercules, CA, USA) following the manufacturer's protocol. Aliquots of protein were separated by 8%-15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidine difluoride membrane (Millipore, Billerica, MA, USA). The membranes were blocked with Tris-buffered saline containing 0.05% Tween 20 and 5% skim milk (Becton Dickinson). After washing, the membranes were incubated with primary antibodies of DR4 (Santa Cruz Biotech, Santa Cruz, CA, USA), DR5 (Koma Biotech, Seoul, Korea), cleaved caspase-3, cleaved caspase-8 and PARP (Cell Signaling, Danvers, MA, USA), Flip (Enzo Life Science) and β-actin (Santa Cruz Biotech). After reaction with horseradish peroxidase conjugated secondary antibodies (Santa Cruz Biotech) bands on the membranes were visualized by an enhanced chemiluminescence system (Thermo Scientific, Rockford, IL, USA) following the manufacturer's suggested procedure. The density of respective bands was analyzed by the chemi-Doc XRS imaging system (Bio-Rad). The data were presented as % of controls.
The data are presented as mean ± SD. Statistical analysis was conducted using one-way analysis of variance (ANOVA) followed by Duncan's multiple range test for a post hoc comparison by using the Statistical Package for Social Science (SPSS 17.0) statistical software. Statistical significance was set at P < 0.05.
To determine whether I3C and Genistein can sensitize endometrial cancer, Ishikawa and HEC1A cells were grown in presence of I3C, Genistein and TRAIL for 24 hr. I3C and Genistein did not show significant cell death alone or in combination. TRAIL only also could not affect in cell viability. However, three-combination (I3C and Genistein with TRAIL) treatment induced significant suppression of cell proliferation in Ishikawa and HEC1A (data not shown). The finding indicated the potency of three-combination over the endometrial cancer and only Ishikawa cells were selected for the further experiments (Fig. 1).
Flow cytometric analysis of cell cycle was performed to examine the mechanism of inhibition of cell growth following I3C, Genistein and TRAIL treatment. There was no significant alternation in the cell cycle population in individual or two-combination treatment. However, remarkable increase in sub-G1 arrest was observed in the combination of three drugs. The treatment increased the ratio of sub-G1 fraction from 5.25% (DMSO control) to 44% (I3C Genistein and TRAIL). Consequently, the G0/G1phase decreased from 55.5% (DMSO control) to 19.35% (I3C, Genistein and TRAIL) (Fig. 2A). To observe the apoptosis, we examined Ishikawa cells for annexin V-FITC staining. Induction of apoptosis was substantiated by examining the flow cytometry pattern of annexin V-FITC stained. Apoptotic cells accounted for 3.6% (TRAIL), 4.8% (I3C and Genistein), and 13.3% (I3C, Genistein and TRAIL) (Fig. 2B).
In order to study the apoptotic response, we examined the caspase-3 activity with the treatment of I3C, Genistein and TRAIL. The TRAIL alone and combination of I3C and Genistein treatment did not show significant changes. The caspase-3 activity was increased in dose dependent manner when TRAIL was added with I3C and Genistein (Fig. 3A). ELISA analysis showed increase of histone-associated DNA fragments (Fig. 3B). The presence of distinct bands after electrophoresis of genomic DNA of cells has become a hallmark of apoptosis (Fig. 3C).
To show whether potential reduction of cell viability and cell arrest was due to apoptosis, the apoptosis inducing genes were examined. The up-regulated expression of DR4, DR5, cleaved caspase-8 and degradation of Flip was observed in three-combination treatment. In addition, the increased expression of cleavage of caspase-3 and PARP confirmed the caspase cascade in apoptotic signaling pathway (Fig. 4).
Despite the large number of individuals affected by endometrial cancer, the mechanism involved in the pathology of this gynecologic cancer remains elusive. New combination regimens have become the major strategies for cancer treatment and improving response rates. Here, we have presented the evidence of a synergistic effect of I3C, Genistein and TRAIL in endometrial cancer.
In this study, the combination of I3C, Genistein and TRAIL showed efficacy to inhibit cell proliferation and to induce apoptosis in human endometrial cancer cell line Ishikawa whereas potential changes was not observed in individual and two-combination treatment. The present results demonstrated that three combination treatments disturbed cell cycle progression by sub-G1 arrest. Synergistic action of this combination treatment defined a greater therapeutic effect than the single component drug. However, it was previously reported that the co-treatment of I3C and Genistein suppressed the viability of human colon cancer HT-29 cells (11). Ouyang et al. (12) also documented that the Genistein inhibited the proliferation of human ovarian cancer cells in high concentrations. Choi et al. (13) showed that Genistein has antiproliferative activity and causes cell cycle arrest at the G1 or G2/M phase through the regulation of gene expression that controls cell cycle in human ovarian cancer SKOV-3 cells. The difference in result cannot be answered definitively in the present work, but a possible explanation can be outlined based on systemic differences and mechanism of kinase activities.
Previously, it has also been reported that the endometrial carcinoma cell line Ishikawa and KLE did not undergo apoptosis with the TRAIL treatment alone (14). Agreeing with above statement we have evaluated the combination effect of I3C and Genistein with TRAIL. Interestingly, we observed the combination treatment upregulated the DR4 and DR5 receptors. Moreover, the combination treatment enhanced the activation of caspase-8 and suppressed the expression level of Flip. TRAIL bind to the death receptors DR4 and DR5 and process of apoptosis was caspase dependent (15). It is known that activated caspase-8 released into cytoplasm, where it initiates a protease cascade which activates effector caspases (16). In addition, it has also been shown that the caspase-8 cleavage can be inhibited by c-Flip through DISC formation (17, 18). The cellular form of Flip protein blocks the death receptor and induced apoptosis. Flip is involved in the resistance to chemotherapy (19, 20) and high expression of Flip has been correlated with TRAIL resistant tumors including ovarian cancer (21, 22).
Membranous death receptor expression on tumor is prerequisite for drugs to be effective as anticancer agent, but functionally the downstream signaling pathway is of equally important. Our result also demonstrated the elevated level of caspase-3 activity and cleaved PARP protein in I3C, Genistein and TRAIL treatment. However, it was previously documented that I3C induced cell death in prostate cancer cells with the release of cytochrome-c, caspase-3 activation and PARP cleavage in apoptotic cascade (23). Jeon et al. (24) demonstrated I3C augmented TRAIL induced apoptosis through activation of caspase-3, and DR4, DR5 up regulation in TRAIL resistant prostate cancer cell line. Additionally, treatment of Genistein and TRAIL activated the caspase-3 in TRAIL resistant human gastric adenocarcinoma AGS cells (25). The recent studies are correlated with our result that the dietary flavonoids sensitize prostate, hepatoma and HeLa cells to TRAIL mediated apoptosis (26-28).
In conclusion, our current findings are consistent with the hypothesis that the combination treatment can sensitize the cancer cells effectively than the individual therapy. We reported that combination treatment of I3C, Genistein and TRAIL synergistically inhibited the cell growth and induced apoptosis through the upregulation of DR4 and DR5 expressions. These findings also highlight the potential genotoxic effects and anticancer mechanisms of I3C, Genistein and TRAIL to maximize the apoptotic effect against endometrial cancer. However, the detailed molecular pathway involved in this mechanism remains to be further investigated.
Figures and Tables
Fig. 1
Growth inhibitory effect of I3C, Genistein (G) and TRAIL (T) in Ishikawa cells. Cells were treated with DMSO (control) or I3C (50 µM) and Genistein (20 µM) and TRAIL (10, 20, and 50 ng/mL) alone or in combination for 24 hr. Cell viability was measured by MTT assay and the results were expressed in percentage of viable cells. Values are means ± SD. *P < 0.05.
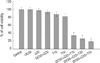
Fig. 2
Effect of I3C, Genistein (G) and TRAIL (T) in cell cycle profile and apoptosis. (A) After treatment with DMSO (control) or I3C (50 µM), Genistein (20 µM) and TRAIL (10, 20, and 50 ng/mL) alone or in combination, cultured endometrial cancer cells were harvested, fixed, stained with PI and analyzed by flow cytometric analysis. The values represent the number of cells in different phases of the cell cycle progression as a percentage of total cells. (B) The dual parameter dots combining annexin V-FITC and PI fluorescence showed apoptotic cells in the lower right quadrant (annexin V+PI-) and necrotic cells in the upper left quadrant (annexin V-PI+).
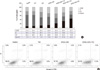
Fig. 3
Effect of I3C, Genistein (G) and TRAIL (T) on caspase-3 activity and DNA fragmentation. Cells were treated with DMSO (control) or I3C (50 µM), Genistein (20 µM) and TRAIL (10, 20, and 50 ng/mL) alone or in combination for 24 hr. (A) Cell lysates were prepared and used to profile caspase-3 activity. (B) DNA fragmentation was analyzed using an ELISA and (C) agarose gel electrophoresis. Values are means ± SD. *P < 0.05.

Fig. 4
Effect of I3C and Genistein followed by TRAIL on apoptosis in Ishikawa cells. Cells were exposed to I3C (50 µM), Genistein (20 µM) and TRAIL (10, 20, and 50 ng/mL) alone or in combination for 24 hr. After treatment, total protein was isolated. Expression of DR4, DR5 cleaved caspase-3, caspase-8, PARP and Flip proteins were analyzed by western blotting. Beta-actin was used as an internal loading control. Each band was quantified by densitometric analysis and presented in a bar graph.

References
1. Gayther SA, Pharoah PD. The inherited genetics of ovarian and endometrial cancer. Curr Opin Genet Dev. 2010. 20:231–238.
2. Homesley HD. Present status and future direction of clinical trials in advanced endometrial carcinoma. J Gynecol Oncol. 2008. 19:157–161.
3. Frantz S. Drug discovery: playing dirty. Nature. 2005. 437:942–943.
4. Wang TT, Milner MJ, Milner JA, Kim YS. Estrogen receptor alpha as a target for indole-3-carbinol. J Nutr Biochem. 2006. 17:659–664.
5. Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008. 269:226–242.
6. Oki T, Sowa Y, Hirose T, Takagaki N, Horinaka M, Nakanishi R, Yasuda C, Yoshida T, Kanazawa M, Satomi Y, et al. Genistein induces Gadd45 gene and G2/M cell cycle arrest in the DU145 human prostate cancer cell line. FEBS Lett. 2004. 577:55–59.
7. Okura A, Arakawa H, Oka H, Yoshinari T, Monden Y. Effect of genistein on topoisomerase activity and on the growth of [Val 12]Ha-ras-transformed NIH 3T3 cells. Biochem Biophys Res Commun. 1988. 157:183–189.
8. Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999. 104:155–162.
9. Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003. 22:8628–8633.
10. Kelley SK, Harris LA, Xie D, Deforge L, Totpal K, Bussiere J, Fox JA. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther. 2001. 299:31–38.
11. Nakamura Y, Yogosawa S, Izutani Y, Watanabe H, Otsuji E, Sakai T. A combination of indol-3-carbinol and genistein synergistically induces apoptosis in human colon cancer HT-29 cells by inhibiting Akt phosphorylation and progression of autophagy. Mol Cancer. 2009. 8:100.
12. Ouyang G, Yao L, Ruan K, Song G, Mao Y, Bao S. Genistein induces G2/M cell cycle arrest and apoptosis of human ovarian cancer cells via activation of DNA damage checkpoint pathways. Cell Biol Int. 2009. 33:1237–1244.
13. Choi EJ, Kim T, Lee MS. Pro-apoptotic effect and cytotoxicity of genistein and genistin in human ovarian cancer SK-OV-3 cells. Life Sci. 2007. 80:1403–1408.
14. LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003. 10:66–75.
15. Dolcet X, Llobet D, Pallares J, Rue M, Comella JX, Matias-Guiu X. FLIP is frequently expressed in endometrial carcinoma and has a role in resistance to TRAIL-induced apoptosis. Lab Invest. 2005. 85:885–894.
16. Srivastava RK. TRAIL/Apo-2L: mechanisms and clinical applications in cancer. Neoplasia. 2001. 3:535–546.
17. Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schröter M, Burns K, Mattmann C, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997. 388:190–195.
18. Xiao C, Yang BF, Asadi N, Beguinot F, Hao C. Tumor necrosis factor-related apoptosis-inducing ligand-induced death-inducing signaling complex and its modulation by c-FLIP and PED/PEA-15 in glioma cells. J Biol Chem. 2002. 277:25020–25025.
19. Abedini MR, Qiu Q, Yan X, Tsang BK. Possible role of FLICE-like inhibitory protein (FLIP) in chemoresistant ovarian cancer cells in vitro. Oncogene. 2004. 23:6997–7004.
20. Safa AR, Day TW, Wu CH. Cellular FLICE-like inhibitory protein (C-FLIP): a novel target for cancer therapy. Curr Cancer Drug Targets. 2008. 8:37–46.
21. Tomek S, Horak P, Pribill I, Haller G, Rössler M, Zielinski CC, Pils D, Krainer M. Resistance to TRAIL-induced apoptosis in ovarian cancer cell lines is overcome by co-treatment with cytotoxic drugs. Gynecol Oncol. 2004. 94:107–114.
22. Lane D, Cartier A, L'Espérance S, Côté M, Rancourt C, Piché A. Differential induction of apoptosis by tumor necrosis factor-related apoptosis-inducing ligand in human ovarian carcinoma cells. Gynecol Oncol. 2004. 93:594–604.
23. Weng JR, Tsai CH, Kulp SK, Wang D, Lin CH, Yang HC, Ma Y, Sargeant A, Chiu CF, Tsai MH, et al. A potent indole-3-carbinol derived antitumor agent with pleiotropic effects on multiple signaling pathways in prostate cancer cells. Cancer Res. 2007. 67:7815–7824.
24. Jeon KI, Rih JK, Kim HJ, Lee YJ, Cho CH, Goldberg ID, Rosen EM, Bae I. Pretreatment of indole-3-carbinol augments TRAIL-induced apoptosis in a prostate cancer cell line, LNCaP. FEBS Lett. 2003. 544:246–251.
25. Jin CY, Park C, Cheong J, Choi BT, Lee TH, Lee JD, Lee WH, Kim GY, Ryu CH, Choi YH. Genistein sensitizes TRAIL-resistant human gastric adenocarcinoma AGS cells through activation of caspase-3. Cancer Lett. 2007. 257:56–64.
26. Szliszka E, Krol W. Soy isoflavones augment the effect of TRAIL-mediated apoptotic death in prostate cancer cells. Oncol Rep. 2011. 26:533–541.
27. Jin CY, Park C, Moon SK, Kim GY, Kwon TK, Lee SJ, Kim WJ, Choi YH. Genistein sensitizes human hepatocellular carcinoma cells to TRAIL-mediated apoptosis by enhancing Bid cleavage. Anticancer Drugs. 2009. 20:713–722.
28. Szliszka E, Czuba ZP, Jernas K, Król W. Dietary flavonoids sensitize HeLa cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Int J Mol Sci. 2008. 9:56–64.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download