Abstract
Our objective was to evaluate the relationship between intrauterine exposure to cadmium and the presence of atopic dermatitis in infants 6 months of age, adjusted for covariates including exposure to other heavy metals. The present research is a component of the Mothers' and Children's Environmental Health (MOCEH) study, a multi-center birth cohort project conducted in Korea. Study subjects were restricted to pregnant women in whom cadmium and lead levels were measured at delivery and whose infants were assessed for the presence of atopic disease at 6 months of age. The odds ratio (OR) for the presence of atopic dermatitis in 6-month-old infants whose cord blood had elevated cadmium levels, after adjustment for other covariates, was 2.350 (95% CI, 1.126-4.906). The OR for the presence of atopic dermatitis in infants whose cord blood had elevated lead levels was not significant. In the present study, the cord blood cadmium level was significantly associated with the presence of atopic dermatitis in 6-month-old infants; this was not true of the cord blood lead level. To the best of our knowledge, this is the first prospective study to show a relationship between prenatal exposure to cadmium and atopic dermatitis in infancy.
The prevalence of atopic dermatitis among children, especially infants, is rising in Korea (1, 2). Atopic dermatitis in infants commences in the first months of life, usually at about 3 months of age. This highlights the need to investigate the nature of prenatal and very early life events that may trigger the onset of allergic march (3). Many environmental contaminants are known to cause atopic dermatitis. Common aeroallergens include indoor/outdoor allergens and pollutants generated by traffic (4-6). Epidemiologic observations on the levels of urban industrial pollution across eastern Germany have strongly suggested that variation in the levels of exposure to metal-rich pollutants that include cadmium or lead largely explain regional variations noted in the prevalence of allergic sensitization in children (7). Sensitization increases as the metal levels of fine particulate matter rise (8).
Cadmium and lead are toxic, pervasive in the environment, and accumulate in the body over a lifetime. The metals contribute to oxidative stress and inhibit DNA repair processes (9-12) Cadmium (13) and lead (14-16) adversely affect the immune system. Exposure to these metals may alter the immune system in a manner triggering atopic dermatitis and asthma. A few studies on the relationship between intrauterine exposure to lead and development of atopic disease in infancy have appeared (17). However, no study on a possible association between intrauterine exposure to cadmium and development of atopic dermatitis in infancy has been conducted yet, to our knowledge.
Our hypothesis was that intrauterine exposure to cadmium was a risk factor for development of atopic dermatitis in infants. Our objective was to evaluate the relationship between exposure to heavy metals and the prevalence of atopic dermatitis in infants 6 months of age, adjusted for the possibly confounding effects of other covariates including the levels of other heavy metals.
The present project is a component of the Mothers' and Children's Environmental Health (MOCEH) study (18), a multicenter birth cohort project conducted in Korea. Our study sample was composed of women in weeks 12-28 of normal pregnancy, with a single fetus, who registered at their local MOCEH study center between August 2006 and December 2009. The study subjects were all pregnant women in whom cadmium and lead levels were assessed at delivery, and development of atopic disease was assessed in infants 6 months of age. Singleton children of 637 mothers were analyzed. All participants were interviewed by trained personnel. Upon enrolment, a detailed questionnaire was used to obtain information on demographics, socioeconomic factors, residential characteristics, medical and reproductive history, exposure to occupational hazards, alcohol consumption, nutritional habits, and exposure to secondhand smoke in the home. Atopic dermatitis was considered present if the mother answered the question: "Were you ever told by a doctor that your child had atopic dermatitis?" in the affirmative. Each questionnaire was completed on the day on which the blood sample was collected.
Cord blood (15 mL) was obtained from a cord site close to the infant, and the levels of lead, cadmium, IgE, and IL-10; and eosinophil number, were measured. Blood was drawn into standard commercial evacuated tubes (Vacutainer®) containing sodium heparin. Blood samples were frozen and stored at -20℃. Prior to analysis, samples were allowed to attain room temperature and were thoroughly vortexed after thawing. Blood samples (each 0.1 mL) were diluted with 1.8 mL of matrix modifier reagent (containing Triton X-100 and ammonium phosphate). Cadmium and lead levels were measured using graphite furnace atomic absorption spectrometry featuring Zeeman background correction (Perkin Elmer AAS800). All metal analysis was performed by the Neodin Medical Institute; the laboratory is certified by the Korean Ministry of Health and Welfare. Commercial reference materials (Lyphochek® Whole Blood Metals Control; Bio-Rad, Hercules, CA, USA) were used for internal quality assurance and as controls. In terms of external quality assurance and control, the Institute participates in both the German External Quality Assessment Scheme operated by Friedrich-Alexander University and the Quality Assurance Program operated by the Korea Occupational Safety and Health Agency. The Institute has also been certified by the Ministry of Employment and Labor as a designated laboratory for analysis of specific materials, including heavy metals and particular organic chemicals. The method detection limits for blood cadmium and lead in the present study were 0.056 µg/L and 0.12 µg/dL, respectively. Only one sample contained a lead level below the detection limit and we considered the level in that sample to be the detection limit divided by the square root of 2 (19). Eosinophil number, and serum total IgE and serum IL-10 levels, were measured as markers of atopy.
The levels of serum total IgE and IL-10, and eosinophil counts, were transformed into natural logarithms, to normalize distributions. Data are expressed as means with standard deviations (continuous variables) or as numbers with percentages (categorical variables). The characteristics of study subjects with respect to atopic status were analyzed using the chi-square test, the t-test, or analysis of variance (ANOVA), as appropriate. All data were analyzed by SPSS software, version 18.0 (SPSS, Chicago, IL, USA). All testing for statistical significance was two-sided, with an α-error of 0.05. The adjusted odds ratios (ORs) (with 95% CIs) for the prevalence of atopic dermatitis in 6-month-old infants of mothers with elevated blood cadmium or lead levels were calculated by logistic regression analysis. Covariates used during adjusted OR calculations included those exhibiting a (borderline) statistically significant difference between mothers of infants with and without atopic dermatitis. Such factors included educational level, a history of atopic disease, parity number, number of family members, gender of the infant, and duration of feeding with breast milk. In addition, we included covariates of biological significance, including a history of passive smoking and cord blood IgE level. Other covariates examined included basic demographic characteristics such as the age and weight of the pregnant mother, and family income.
Prior to the enrolment, all study participants were provided for written informed consent. The study protocol and the consent form were approved by the institutional review boards of Ewha Womans University (Seoul) (approval No. 12-07B-15); Dankook University Hospital (Cheonan) (approval No. 2011-09-0340); and Ulsan University Hospital (Ulsan) (approval No. 06-29); all three hospitals are located in the Republic of Korea.
Table 1 shows the overall characteristics of study infants grouped by atopic status. Maternal educational level and maternal history of atopic disease, differed significantly when data from mothers of atopic and non-atopic infants were compared. However, no other statistically significant difference between such mothers was noted (Table 1).
The overall mean level of cord blood cadmium was 0.71 µg/L and was slightly lower in the cords of atopic compared to non-atopic infants. The overall mean level of cord blood lead level was 1.01 µg/dL (Table 2) and did not differ between atopic and non-atopic infants. The geometric means of the cord blood IgE level, the eosinophil count, and the IL10 level did not significantly differ between atopic and non-atopic infants.
The OR and 95% CI values of cord blood cadmium level for the presence of atopic dermatitis in 6 month-old infants were 2.350 (1.126-4.906) (model 1) after adjustment for other covariates. The OR remained significant after further adjustment for a history of passive smoking (for which the OR was not significant) (model 2). The OR continued to be significant after further adjustment for cord blood lead level (for which the OR [95% CI] was 1.045 [0.602-1.811]) (model 3). The OR for cord blood IgE level was not significant (model 4) (Table 3).
In the present study, cord blood cadmium level was found to be significantly associated with the development of atopic dermatitis in 6-month-old infants. Cadmium is a toxic heavy metal. Tobacco and food contamination represent the main sources of non-occupational exposure in the general population (20-22).
The overall mean level of cord blood cadmium was 0.71 µg/L in the present study. This is similar to the levels of 0.6 µg/L and 0.78 µg/L measured in Saudi Arabia and India, respectively (23, 24), but higher than the 0.21 µg/L and 0.021 µg/L reported in Belgium and Germany, respectively (25, 26). The level was much lower than the 5 µg/L threshold set by the Biological Exposure Index (BEI) of the American Conference of Governmental Industrial Hygienists (ACGIH) as safe in workers (27). The most common sources of environmental cadmium exposure are the diet and tobacco. As cadmium is more efficiently absorbed via the lungs than via the gastrointestinal tract (28), smoking poses a serious risk of exposure. A single cigarette usually contains 1-2 µg cadmium (29). In the present study, the overall mean level of cord blood cadmium did not differ significantly between mothers with and without a history of passive smoking (0.72 µg/L vs 0.71 µg/L). Such a history was not associated with development of atopic dermatitis. The OR of cord blood cadmium for the development of atopic dermatitis in 6-month-old infants remained significant after adjustment for a history of passive smoking. Thus, atopic dermatitis seems to be associated with absorption of cadmium in the gastrointestinal tract, and not with smoking.
We found that the cord blood cadmium level was significantly associated with the presence of atopic dermatitis in 6-month-old infants, after adjustment for various covariates. These findings are compatible with those of a previous study by Razi et al. (30), who found that cadmium exposure aggravated the asthmatic status of children. Environmental exposure to metals can modulate immune homeostasis, and cause inadequate or excessive production of inflammatory cytokines (31). Hemdan et al. (32) showed that short-term exposure of cells stimulated with a monoclonal antibody to low levels of cadmium markedly inhibited secretion of interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ compared to production of IL-4 and IL-10. This indicates that the immune response shifts from type-1 helper T cells (Th1) to type-2 helper T cells (Th2) under such conditions. The authors speculated that, in agreement with literature data, such a shift may increase the incidence of allergic disease. We measured the eosinophil number, and the levels of total IgE and IL-10 as atopic markers, where these atopic markers were not associated with development of atopic dermatitis in the present study. Although IgE antibodies play a pivotal role in atopic dermatitis, cord blood IgE may be less useful test for predicting atopic dermatitis. Munasir et al. (33) reported that only 18.9% of the subjects with atopic dermatitis showed high levels of cord blood IgE ( > 1.2 IU/µL) in the first 6 months of life. Some studies have shown that elevated cord blood IgE increases sensitization to aeroallergens, but it does not always lead to a concomitant increase in respiratory or other allergic disorders (34-36). In addition, cord blood IgE levels may be low due to the infant's immature immune system (34). It seems that cord blood eosinophil is not a good predictor for developing atopic disease (37). An impaired Th1 and T regulatory cell responses at birth may contribute to the development of atopic dermatitis (38), but cord blood IL-10 was not significantly associated with development of atopic dermatitis in the present study. Thus, the mechanism of development of atopic dermatitis associated with exposure to cadmium remains to be clarified in infants.
Earlier data suggested that lead exposure may alter components of the immune system known to be involved in the development of atopic dermatitis and asthma (14, 15). Both Lutz et al. (39) and Sun et al. (40) reported a significant positive relationship between blood lead and IgE levels in studies on children. Jedrychowski et al. (17) showed that intrauterine exposure to lead may enhance sensitization to common inhalant allergens in early childhood. However, the cord blood lead level was not significantly associated with development of atopic dermatitis or the cord blood IgE level in the present study. The observed discrepancies between the data of our study and those of others may be attributable in part to differences in target populations (infants vs children or adults) (17, 39, 40); the diseases studied (atopic dermatitis vs asthma) (17); and/or exposure times (prenatal vs postnatal) (39, 40). Further work is required.
Our present findings have public health implications. The data suggest that low-level prenatal cadmium exposure may be implicated in development of atopic dermatitis in infancy. A reduction in the level of exposure of pregnant women to environmental cadmium may decrease the prevalence of atopic dermatitis (which is currently rising worldwide).
The strengths of the present study are, first, that we used a prospective cohort design commencing in the prenatal period. Thus, we sought to rule out the possibility that cadmium exposure levels might be erroneously classified. Further, we sought to eliminate the selection bias that may have occurred if parents concerned with the health of their children were principally interested in volunteering to participate in our study.
Our study had several limitations. First, although we obtained information on the home environment (via a questionnaire), we did not directly assess this environment. For example, we did not directly measure aeroallergen concentrations in house dust, or indoor levels of chemicals of interest. Therefore, we cannot completely rule out potentially confounding effects of the home environment. Second, development of atopic dermatitis was based on parental reporting and therefore subject to a lack of standardization, misclassification, or recall bias; over-reporting might have occurred. Finally, the association between cord blood cadmium level and development of atopic dermatitis does not appear to be strong; the OR is low. The mechanism of development of atopic dermatitis associated with exposure to cadmium remains unclear. Future work should explore whether the effects described here can be replicated using a similar study design but with measurement of a wider spectrum of prenatal indoor and outdoor environmental hazards.
In conclusion, increased levels of cord blood cadmium were observed in 6-month-old infants with atopic dermatitis. Further studies are warranted to confirm this association.
Figures and Tables
Table 1
Characteristics of the study population, grouped by the atopic status of 6-month-old infants

Table 3
Odds ratios (ORs), with 95% confidence intervals (CIs), for development of atopic dermatitis in 6-month-old infants, after adjustment for the confounding effects of covariates (n=446)
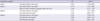
*P < 0.05. Adjusted for age, weight, a history of atopic disease, educational level of the mother, gender of the infant, family income, number of individuals in the family, parity, and duration of breast feeding (Models 1-4). To construct Model 2, a history of passive smoking in pregnant women was added as a covariate additional to those of Model 1. For Model 3, cord blood lead level was added to the covariates of Model 2. For Model 4, cord blood IgE level was added to the covariates of Model 3.
ACKNOWLEDGMENTS
The authors declare that there are no conflicts of interest. No author declares any competing financial interest.
References
1. Oh JW, Pyun BY, Choung JT, Ahn KM, Kim CH, Song SW, Son JA, Lee SY, Lee SI. Epidemiological change of atopic dermatitis and food allergy in school-aged children in Korea between 1995 and 2000. J Korean Med Sci. 2004. 19:716–723.
2. Yu JS, Lee CJ, Lee HS, Kim J, Han Y, Ahn K, Lee SI. Prevalence of atopic dermatitis in Korea: analysis by using national statistics. J Korean Med Sci. 2012. 27:681–685.
3. Taïeb A, Boralevi F. Ring J, Przybilla B, Ruzicka T, editors. Atopic eczema in infants. Handbook of atopic eczema. 2006. 2nd ed. Berlin: Springer;45–60.
4. Schwarze PE, Ovrevik J, Låg M, Refsnes M, Nafstad P, Hetland RB, Dybing E. Particulate matter properties and health effects: consistency of epidemiological and toxicological studies. Hum Exp Toxicol. 2006. 25:559–579.
5. Rusznak C, Jemkins S, Mills PR, Sapsford RJ, Devaglia JL, Davies RJ. Mechanism of pollution-induced allergy and asthma. Rev Fr Allergol Immunol Clin. 1998. 38:s80–s90.
6. Devalia JL, Rusznak C, Wang J, Khair OA, Abdelaziz MM, Calderón MA, Davies RJ. Air pollutants and respiratory hypersensitivity. Toxicol Lett. 1996. 86:169–176.
7. Heinrich J, Hoelscher B, Wjst M, Ritz B, Cyrys J, Wichmann H. Respiratory diseases and allergies in two polluted areas in East Germany. Environ Health Perspect. 1999. 107:53–62.
8. Gavett SH, Haykal-Coates N, Copeland LB, Heinrich J, Gilmour MI. Metal composition of ambient PM2.5 influences severity of allergic airways disease in mice. Environ Health Perspect. 2003. 111:1471–1477.
9. Factors influencing metabolism and toxicity of metals: a consensus report. Environ Health Perspect. 1978. 25:3–41.
10. Telisman S. Interactions of essential and/or toxic metals and metalloid regarding interindividual differences in susceptibility to various toxicants and chronic diseases in man. Arh Hig Rada Toksikol. 1995. 46:459–476.
11. Jurasović J, Pizent A, Telišman S. Roussel AM, Anderson RA, Favrier AE, editors. Serum selenium in relation to biomarkers of lead in men. 2000. New York: Kluwer Academic/Plenum Publishers;675–678.
12. Pizent A, Jurasović J, Telisman S. Serum calcium, zinc, and copper in relation to biomarkers of lead and cadmium in men. J Trace Elem Med Biol. 2003. 17:199–205.
13. Jelovcan S, Gutschi A, Kleinhappl B, Sedlmayr P, Barth S, Marth E. Effects of low concentrations of cadmium on immunoglobulin E production by human B lymphocytes in vitro. Toxicology. 2003. 188:35–48.
14. Fischbein A, Tsang P, Luo JC, Roboz JP, Jiang JD, Bekesi JG. Phenotypic aberrations of CD3+ and CD4+ cells and functional impairments of lymphocytes at low-level occupational exposure to lead. Clin Immunol Immunopathol. 1993. 66:163–168.
15. Undeger U, Başaran N, Canpinar H, Kansu E. Immune alterations in lead-exposed workers. Toxicology. 1996. 109:167–172.
16. Sata F, Araki S, Tanigawa T, Morita Y, Sakurai S, Katsuno N. Changes in natural killer cell subpopulations in lead workers. Int Arch Occup Environ Health. 1997. 69:306–310.
17. Jedrychowski W, Perera F, Maugeri U, Miller RL, Rembiasz M, Flak E, Mroz E, Majewska R, Zembala M. Intrauterine exposure to lead may enhance sensitization to common inhalant allergens in early childhood: a prospective prebirth cohort study. Environ Res. 2011. 111:119–124.
18. Kim BM, Ha M, Park HS, Lee BE, Kim YJ, Hong YC, Kim Y, Chang N, Roh YM, Kim BN, et al. The Mothers and Children's Environmental Health (MOCEH) Study. Eur J Epidemiol. 2009. 24:573–583.
19. Glass DC, Gray CN. Estimating mean exposures from censored data: exposure to benzene in the Australian petroleum industry. Ann Occup Hyg. 2001. 45:275–282.
20. Hossny E, Mokhtar G, El-Awady M, Ali I, Morsy M, Dawood A. Environmental exposure of the pediatric age groups in Cairo city and its suburbs to cadmium pollution. Sci Total Environ. 2001. 273:135–146.
21. Järup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure: a review of the literature and a risk estimate. Scand J Work Environ Health. 1998. 24:1–51.
22. Pan J, Plant JA, Voulvoulis N, Oates CJ, Ihlenfeld C. Cadmium levels in Europe: implications for human health. Environ Geochem Health. 2010. 32:1–12.
23. Raghunath R, Tripathi RM, Sastry VN, Krishnamoorthy TM. Heavy metals in maternal and cord blood. Sci Total Environ. 2000. 250:135–141.
24. Al-Saleh I, Shinwari N, Mashhour A, Mohamed Gel D, Rabah A. Heavy metals (lead, cadmium and mercury) in maternal, cord blood and placenta of healthy women. Int J Hyg Environ Health. 2011. 214:79–101.
25. Koppen G, Den Hond E, Nelen V, Van De Mieroop E, Bruckers L, Bilau M, Keune H, Van Larebeke N, Covaci A, Van De Weghe H, et al. Organochlorine and heavy metals in newborns: results from the Flemish Environment and Health Survey (FLEHS 2002-2006). Environ Int. 2009. 35:1015–1022.
26. Osman K, Akesson A, Berglund M, Bremme K, Schütz A, Ask K, Vahter M. Toxic and essential elements in placentas of Swedish women. Clin Biochem. 2000. 33:131–138.
27. American Conference of Governmental Industrial Hygienists (ACGIH). Threshold limit values for chemical substances and physical agents and biological exposure indices. 2008. Cincinnati: ACGIH.
28. Taylor J, DeWoskin R, Ennever FK. Toxicological profile for cadmium US Department of Health and Human Services, Public Health Service. 1999. Atlanta: Agency for Toxic Substances and Disease Registry.
29. Weyermann M, Brenner H. Alcohol consumption and smoking habits as determinants of blood lead levels in a national population sample from Germany. Arch Environ Health. 1997. 52:233–239.
30. Razi CH, Akin KO, Harmanci K, Ozdemir O, Abaci A, Hizli S, Renda R, Celik A. Relationship between hair cadmium levels, indoor ETS exposure and wheezing frequency in children. Allergol Immunopathol (Madr). 2012. 40:51–59.
31. Lawrence DA, McCabe MJ Jr. Immunomodulation by metals. Int Immunopharmacol. 2002. 2:293–302.
32. Hemdan NY, Emmrich F, Sack U, Wichmann G, Lehmann J, Adham K, Lehmann I. The in vitro immune modulation by cadmium depends on the way of cell activation. Toxicology. 2006. 222:37–45.
33. Munasir Z, Sastroasmoro S, Djauzi S, Waspadji S, Ramelan W, Aminullah A, Widowati R, Harahap AR, Endaryanto A, Wahidiyat I. The role of allergic risk and other factors that affect the occurrence of atopic dermatitis in the first 6 months of life. Asia Pac Allergy. 2011. 1:73–79.
34. Eiríksson TH, Sigurgeirsson B, Ardal B, Sigfússon A, Valdimarsson H. Cord blood IgE levels are influenced by gestational age but do not predict allergic manifestations in infants. Pediatr Allergy Immunol. 1994. 5:5–10.
35. Edenharter G, Bergmann RL, Bergmann KE, Wahn V, Forster J, Zepp F, Wahn U. Cord blood-IgE as risk factor and predictor for atopic diseases. Clin Exp Allergy. 1998. 28:671–678.
36. Shah PS, Wegienka G, Havstad S, Johnson CC, Ownby DR, Zoratti EM. The relationship between cord blood immunoglobulin E levels and allergy-related outcomes in young adults. Ann Allergy Asthma Immunol. 2011. 106:245–251.
37. Haus M, Hall JM, Heese Hde V, Weinberg EG, Berman D. Cord blood and maternal total eosinophil counts in relation to infant allergy. Pediatr Alllergy Immunol. 1992. 3:23–27.
38. Lehmann I, Herberth G. Cord blood immune status: predicting health or allergy? Allergy. 2012. 67:445–448.
39. Lutz PM, Wilson TJ, Ireland J, Jones AL, Gorman JS, Gale NL, Johnson JC, Hewett JE. Elevated immunoglobulin E (IgE) levels in children with exposure to environmental lead. Toxicology. 1999. 134:63–78.
40. Sun L, Hu J, Zhao Z, Li L, Cheng H. Influence of exposure to environmental lead on serum immunoglobulin in preschool children. Environ Res. 2003. 92:124–128.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download