Abstract
This study was performed to characterize respiratory viral infections in pediatric patients undergoing hematopoietic stem cell transplantation (HSCT). Study samples included 402 respiratory specimens obtained from 358 clinical episodes that occurred in the 116 children of the 175 consecutive HSCT cohort at Seoul National University Children's Hospital, Korea from 2007 to 2010. Multiplex reverse-transcription polymerase chain reactions were performed for rhinovirus, respiratory syncytial virus (RSV), parainfluenza viruses (PIVs), adenovirus, human coronavirus (hCoV), influenza viruses and human metapneumovirus. Viruses were identified in 89 clinical episodes that occurred in 58 patients. Among the 89 clinical episodes, frequently detected viruses were rhinovirus in 25 (28.1%), RSV in 23 (25.8%), PIV-3 in 16 (18.0%), adenovirus in 12 (13.5%), and hCoV in 10 (11.2%). Lower respiratory tract infections were diagnosed in 34 (38.2%). Neutropenia was present in 24 (27.0%) episodes and lymphopenia was in 31 (34.8%) episodes. Sixty-three percent of the clinical episodes were hospital-acquired. Three patients died of respiratory failure caused by respiratory viral infections. Respiratory viral infections in pediatric patients who have undergone HSCT are common and are frequently acquired during hospitalization. Continuous monitoring is required to determine the role of respiratory viruses in immunocompromised children and the importance of preventive strategies.
Respiratory infections are important complications in pediatric patients undergoing hematopoietic stem cell transplantation (HSCT) (1, 2). Broad-spectrum antibiotics are administered in children after HSCT to prevent bacterial pneumonia, and antifungal agents and strict screening systems are used for prophylaxis of fatal fungal infections. These efforts have been effective for improving the survival of HSCT patients.
However, respiratory viral infections are common causes of upper respiratory infection (URI), and the clinical significance of these infections has historically been underestimated in the HSCT population. Respiratory viral infections cause progression from URI to lower respiratory tract infection (LRTI) (3, 4) and are frequently associated with bacterial or fungal co-infections (5, 6). During the past 20 yr, infections due to respiratory viruses have been increasingly recognized as potential causes of severe pneumonia after HSCT, with high morbidity and mortality (7-9).
Many advances have been made in the diagnosis of respiratory viral infections and in the management of these infections. Available methods for virus detection include antigen detection by enzyme-linked immunosorbent assay (ELISA), immunofluorescence (IF) assay, enzymatic detection by chemical reactions and polymerase chain reaction (PCR) amplification (10). PCR assay is now commonly used due to its high specificity and sensitivity (11, 12). While detection of respiratory viral infection has improved due to many available diagnostic tests, therapeutic modalities using specific antiviral agents remain limited (13, 14).
This study was performed to evaluate the prevalence of respiratory viral infections in pediatric HSCT recipients and to characterize the clinical manifestations of respiratory viral infections in children after HSCT.
This study recruited a cohort of 175 children who underwent HSCT at Seoul National University Children's Hospital from January 2007 to March 2010 and were followed for at least 1 yr after HSCT. Clinical and virological data were extracted from a prospectively compiled, integrated database.
Respiratory specimens were obtained during routine patient care. Respiratory samples were collected from patients who visited the emergency room or were hospitalized when eligible subjects had febrile episodes or respiratory symptoms or radiologic abnormalities. In total, 402 respiratory specimens were collected from 358 clinical episodes that occurred in the 116 children of the 175 consecutive HSCT cohort via nasopharyngeal aspiration, transtracheal aspiration, sputum, and bronchoalveolar lavage.
All specimens were tested by multiplex reverse transcription PCR (RT-PCR) for 12 respiratory viruses; rhinovirus, adenovirus, respiratory syncytial virus (RSV) types A and B, parainfluenza virus (PIV) types 1, 2, and 3, influenza virus types A and B, human coronavirus (hCoV) 229E/NL63, OC43 and human metapneumovirus (hMPV). The 2009 H1N1 influenza virus A was excluded in this study. Viral RNA was extracted from each specimen using MagNA pure LC (Roche Diagnostics, Seoul, Korea) according to manufacturer's instructions and PCR amplification was performed using Seeplex™ RV12 ACE detection kit (Seegene, Seoul, Korea).
All hospital records of the subjects were reviewed using the standard form of clinical data to identify respiratory viral infections. To evaluate the clinical factors of URI and development to LRTI, respiratory symptoms (onset of symptoms, duration of fever, chest radiographic findings and abnormal breath sound) and status of patients (age, sex, underlying disease, transplantation method, interval from HSCT, days after hospitalization, and laboratory findings) were retrospectively analyzed in detail.
Fever was defined as measured axillary temperature higher than 38.0℃. Community-acquired infection was defined by onset of symptoms within 48 hr of admission, and hospital-acquired infection was defined by onset of symptoms greater than 48 hr after admission. Clinical diagnosis was classified as URI and LRTI. Neutropenia was defined as absolute neutrophil count below 500 cells/µL, and lymphopenia was defined as absolute lymphocyte count below 200 cells/µL.
Each clinical episode was defined as an independent clinical episode when symptoms were newly developed or different respiratory viruses were detected in two consecutive samples that were obtained at least 48 hr apart. When the same virus was detected during the period in which initial symptoms and signs were unresolved, the specific virus was regarded as the detection during a single clinical episode. Co-detection was defined as a detection of ≥ 2 respiratory viruses.
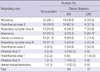
The clinical characteristics of 175 patients who underwent HSCT consecutively during the study period are summarized in Table 1. One hundred-two patients (58.3%) were male and 73 patients (41.7%) were female. The median age was 9.8 yr (range 1.0-25.9 yr). The donor type was autologous in 79 (45.1%) patients and allogeneic in 96 (54.9%) patients. Differences in transplant type were not significant between virus-positive and virus-negative groups (34.1% vs 32.3%, P = 0.792). Differences in underlying disease and sex were not significant in virus detection.
Respiratory viruses were identified in 112 (27.9%) respiratory samples (83 nasopharyngeal aspirates, 14 sputum specimens, 14 transtracheal aspirates, and 1 bronchoalveolar lavage respectively) from the 402 samples that were obtained from 116 patients. Respiratory viral infections were documented in 89 separate clinical episodes except for 23 samples that were persistently positive for the same virus in the same clinical episode.
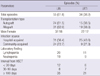
Respiratory viruses were identified from 89 episodes (28.2%) that occurred in 58 children (49.6%). Rhinovirus was detected most frequently, in 25 episodes (28.1%), RSV in 23 (25.8%), PIV-3 in 16 (18.0%), adenovirus in 12 (13.5%), hCoV in 10 (11.2%), PIV-2 in 4 (4.5%), influenza virus B in 3 (3.4%), PIV-1 in 2 (2.2%), influenza virus A in 1 (1.1%) and hMPV in 1 (1.1%) (Table 2). Among 89 clinical episodes, co-detection occurred in 8 episodes (9.0%). Rhinovirus was the most common in co-detection, in 6 of 8 episodes.
The monthly distributions of detected respiratory viruses are shown in Fig. 1. Rhinovirus was detected year around and RSV was prevalent between December and March (82.5% of total isolates). PIV-3 was prevalent between June and September (87.5% of total isolates).
In 89 clinical episodes in which viruses were documented, cough was the most common symptom (79.8%) at the time of laboratory diagnosis of respiratory viral infections. Fever was found in 60 episodes (67.4%) and the median duration of fever was 2 days (range: 0-43 days). Sputum (52.8%) and rhinorrhea (38.2%) were less common.
HSCT patients were followed-up for at least one year and median follow-up period was 1.91 yr (range from 1 yr to 4.17 yr). Overall, 18 episodes (20.2%) occurred within 30 days from the HSCT, 56 (62.9%) occurred after 100 days following HSCT and 15 (16.9%) occurred in the interim. With regard to nosocomial acquisition of respiratory infections, 37% (33 of 89 episodes) were community-acquired and 63% (56 of 89 episodes) were hospital-acquired.
At the time of laboratory diagnosis of respiratory viral infection, 25.8% of patients had neutropenia and 34.5% had lymphopenia. The median absolute neutrophil count (ANC) was 1,920 cells/µL (range: 0-11,585 cells/µL) and the median absolute lymphocyte count (ALC) was 529 cells/µL (range: 0-5,303 cells/µL).
The proportions of each virus in the clinical diagnosis are summarized in Table 2. Rhinovirus (76%, P = 0.091) and hCoV (90.0%, P = 0.053) seemed to present more commonly as URI than LRTI, but the results showed no clinical significance. Adenovirus was more commonly associated with LRTI (66.7%) than URI (33.3%) (P = 0.030).
Clinical diagnoses were URI in 55 (61.8%) episodes and LRTI in 34 (38.2%) episodes. The comparisons of clinical characteristics between URI and LRTI are shown in Table 3. Transplantation type, sexual factor, and laboratory findings were not significantly different between URI and LRTI.
As shown in Table 4, 66 episodes (74.2%) recovered without pulmonary complications or intervention and 23 episodes (25.8%) developed complications. Prophylactic or therapeutic antibiotics were used in 78 episodes (87.6%). Oxygen was required in 21 episodes (23.6%). Pleural effusion was detected on chest radiograph in 15 episodes (16.9%). Mechanical ventilation in the intensive care unit was used in 11 episodes, and the mean duration of ventilator use was 18.4 days.
Among 89 clinical episodes which were developed in 58 HSCT patients, 3 patients died of respiratory failure directly related to respiratory viral infection. One patient who underwent bone marrow transplantation (BMT) for relapsed acute lymphocytic leukemia died of acute respiratory distress after an adenoviral infection. Another patient who underwent peripheral blood stem cell transplantation (PBSCT) for acute myeloplastic leukemia died of respiratory failure, again after an adenoviral infection. Immunosuppressive agents were discontinued as respiratory symptoms aggravated. Both patients were to receive treatment with Cidofovir 5 mg/kg weekly, but after 1 administered dose, they deceased. A third patient, who underwent PBSCT, was hospitalized for relapsed acute myelodysplastic leukemia. This patient had been suffering from chronic graft-versus-host disease (GVHD) and was being treated with prednisolone. A PIV-3 infection occurred during immunosuppressant treatment for GVHD. After PIV-3 was confirmed, her respiratory distress aggravated, eventually leading to death.
This study illustrated that respiratory viral infections are common among children who have undergone HSCT. In our study, respiratory viruses were identified in 27.9% of respiratory samples. Rhinovirus was the most common virus (28.1%), PIV-3 was second (18.0%), and RSV-A was third (14.6%). Lower respiratory tract infections were diagnosed in 38.2% and hospital-acquired infection episodes were in 63.0%. Many recent studies have reported that community respiratory viruses, particularly RSV and PIV, are significant causes of morbidity and mortality among HSCT recipients (15, 16). However, the study for respiratory viral infection of previously healthy children in similar area revealed different results that RSV (23.7%) was the most common detected virus, and as followed adenovirus (6.8%), PIV-3 (6.2%), rhinovirus (5.8%), and hMPV (4.7%) (17). Relatively low prevalence of rhinovirus and human coronavirus (2.9%) implied that the differences exist between healthy population and children who have undergone HSCT.
A review of cases of nonbacterial pneumonia among HSCT recipients from 1969 to 1979 did not report any community-acquired respiratory viral infections (18). However, the prevalence of respiratory viral infections after HSCT has been increasingly reported ranging from 16.5% to 38% (7, 19, 20). In our study, respiratory viruses were detected in 58 (33.1%) HSCT patients.
The prevalence of respiratory viral infections among HSCT patients depends on the diagnostic method used. We used real time RT-PCR method because of recent common usage and excellent sensitivity in comparison with conventional methods such as viral culture and immunofluorescent stain of viral antigen. The reported frequency of RSV infections has varied from 35% to 49%; influenza virus infections from 11% to 45%; PIV infections from 8% to 30%; and rhinovirus infections from 4% to 9% (15, 21). This study demonstrated that rhinovirus was the most frequently detected infection. However, the diagnostic technologies used in each study were different. Therefore, the results of our study cannot be directly compared with those of other studies. A prospective study using RT-PCR shows that rhinovirus was the most common isolate (37.0%) in HSCT patients (22).
Similar to community epidemiology, PIV-3 is common from June to September and RSV is common from December to March. These results were not significantly different from most studies (20, 23).
One of the important findings from this study is that the great proportion (63.0%) of respiratory viral infections was acquired while HSCT patients were hospitalized. High nosocomial infection rates remind us of the importance of contact or droplet precautions and visitor limitation. In most cases, specific antiviral agents are difficult to use clinically, so prevention is the most useful method to reduce respiratory viral infection.
Clinically, URI is the most common predominant presentation of respiratory viral infection and usually resolves without therapy. In our study, 61.8% of respiratory viral infections were limited to URI and 38.2% of those progressed to LRTI. In a European study, 55% of total respiratory viral infection was reported as LRTI (15). A prospective study in adults with HSCT or hematologic malignancy reported that during the follow-up period, 18% of patients had pneumonia or had progression to pneumonia (23). Lymphopenia (≤ 200 cells/µL) was known to be a risk factor for progression to LRTI (21). But in our study, lymphopenia was not significant to the progression to LRTI.
In this study, we found three fatal cases among 89 clinical episodes which were developed in 58 HSCT patients. In these fatal cases, basal lung condition of patients was poor when respiratory distress occurred. However, respiratory viral infection triggered respiratory dysfunction, and their death was probably related to respiratory viral infection. Many other studies reported various mortality and fatality. RSV related mortality in children treated for acute myeloid leukemia was 10% (24), and one report demonstrated PIV-associated fatality of 27% among HSCT recipients (25). Other studies reported mortality rates from respiratory viral infections among HSCT recipients of 0.5% and 0.6% (15, 20).
There are few antiviral therapeutic options against respiratory viruses except influenza virus in HSCT patients. Neuramidase inhibitors, oseltamivir and zanamivir, are known to resolve influenza symptoms in immunocompromised patients (26). M2 inhibitors, amantadine and rimantadine, have been used in some centers, but are limited to influenza A and have many side effects including antiviral resistance (27). The primary therapy for RSV that has been the best studied is aerosolized ribavirin. However, similar to other viral agents, its use has the problem of cost-effectiveness. Researchers reported that aerosolized ribavirin resulted in a decrease in viral load but no difference in the progression to pneumonia (28). Aerosolized and oral ribavirin are used for therapy of PIV infection in transplant recipients with conflicting results (29, 30). Various treatments for adenovirus have been tried including high-dose intravenous immunoglobulin, ribavirin and cidofovir. However, the clinical efficacy of these agents remains unclear. These therapeutic limitations emphasize importance of the prevention strategy and strict infection control program in the hospital.
This study included a relatively large number of patients enrolled at one center. However, our retrospective study dependent on medical records has some limitations for clinical evaluation. In particular, risk factors or prognosis for respiratory viral infection such as conditioning regimen, presence of graft-versus-host disease, taking history of immunosuppressant agents, and separation principle of hospitalized patients cannot be investigated. Despite these limitations, this study is meaningful considering aspects that show the common prevalence of respiratory viral infection in HSCT children. This effort aids investigation of the clinical features and epidemiologic character of virus infections in this patient population.
This study has demonstrated that respiratory viral infection is relatively high in HSCT patients and more than half of the respiratory infections were acquired during hospitalization. These findings emphasize importance of the preventive strategies against respiratory viral infection in HSCT patients at high risk.
Figures and Tables
References
1. Walter EA, Bowden RA. Infection in the bone marrow transplant recipient. Infect Dis Clin North Am. 1995. 9:823–847.
2. Carlson K, Backlund L, Smedmyr B, Oberg G, Simonsson B. Pulmonary function and complications subsequent to autologous bone marrow transplantation. Bone Marrow Transplant. 1994. 14:805–811.
3. Henderson FW, Clyde WA Jr, Collier AM, Denny FW, Senior RJ, Sheaffer CI, Conley WG 3rd, Christian RM. The etiologic and epidemiologic spectrum of bronchiolitis in pediatric practice. J Pediatr. 1979. 95:183–190.
4. Yun BY, Kim MR, Park JY, Choi EH, Lee HJ, Yun CK. Viral etiology and epidemiology of acute lower respiratory tract infections in Korean children. Pediatr Infect Dis J. 1995. 14:1054–1059.
5. Whimbey E, Bodey GP. Viral pneumonia in the immunocompromised adult with neoplastic disease: the role of common community respiratory viruses. Semin Respir Infect. 1992. 7:122–131.
6. Bakaletz LO. Viral potentiation of bacterial superinfection of the respiratory tract. Trends Microbiol. 1995. 3:110–114.
7. Whimbey E, Champlin RE, Couch RB, Englund JA, Goodrich JM, Raad I, Przepiorka D, Lewis VA, Mirza N, Yousuf H, et al. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin Infect Dis. 1996. 22:778–782.
8. Ljungman P. Prevention and treatment of viral infections in stem cell transplant recipients. Br J Haematol. 2002. 118:44–57.
9. McCann S, Byrne JL, Rovira M, Shaw P, Ribaud P, Sica S, Volin L, Olavarria E, Mackinnon S, Trabasso P, et al. Outbreaks of infectious diseases in stem cell transplant units: a silent cause of death for patients and transplant programmes. Bone Marrow Transplant. 2004. 33:519–529.
10. Falsey AR, McCann RM, Hall WJ, Criddle MM. Evaluation of four methods for the diagnosis of respiratory syncytial virus infection in older adults. J Am Geriatr Soc. 1996. 44:71–73.
11. Osiowy C. Direct detection of respiratory syncytial virus, parainfluenza virus, and adenovirus in clinical respiratory specimens by a multiplex reverse transcription-PCR assay. J Clin Microbiol. 1998. 36:3149–3154.
12. van Kraaij MG, van Elden LJ, van Loon AM, Hendriksen KA, Laterveer L, Dekker AW, Nijhuis M. Frequent detection of respiratory viruses in adult recipients of stem cell transplants with the use of real-time polymerase chain reaction, compared with viral culture. Clin Infect Dis. 2005. 40:662–669.
13. Sparrelid E, Ljungman P, Ekelof-Andstrom E, Aschan J, Ringden O, Winiarski J, Wahlin B, Andersson J. Ribavirin therapy in bone marrow transplant recipients with viral respiratory tract infections. Bone Marrow Transplant. 1997. 19:905–908.
14. Kumar D, Humar A. Respiratory viral infections in transplant and oncology patients. Infect Dis Clin North Am. 2010. 24:395–412.
15. Ljungman P, Ward KN, Crooks BN, Parker A, Martino R, Shaw PJ, Brinch L, Brune M, De La Camara R, Dekker A, et al. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001. 28:479–484.
16. Nichols WG, Gooley T, Boeckh M. Community-acquired respiratory syncytial virus and parainfluenza virus infections after hematopoietic stem cell transplantation: the Fred Hutchinson Cancer Research Center experience. Biol Blood Marrow Transplant. 2001. 7:Suppl. 11S–15S.
17. Choi EH, Lee HJ, Kim SJ, Eun BW, Kim NH, Lee JA, Lee JH, Song EK, Kim SH, Park JY, et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000-2005. Clin Infect Dis. 2006. 43:585–592.
18. Meyers JD, Flournoy N, Thomas ED. Nonbacterial pneumonia after allogeneic marrow transplantation: a review of ten years' experience. Rev Infect Dis. 1982. 4:1119–1132.
19. Kuypers J, Campbell AP, Cent A, Corey L, Boeckh M. Comparison of conventional and molecular detection of respiratory viruses in hematopoietic cell transplant recipients. Transpl Infect Dis. 2009. 11:298–303.
20. Machado CM, Boas LS, Mendes AV, Santos MF, da Rocha IF, Sturaro D, Dulley FL, Pannuti CS. Low mortality rates related to respiratory virus infections after bone marrow transplantation. Bone Marrow Transplant. 2003. 31:695–700.
21. Chemaly RF, Ghosh S, Bodey GP, Rohatgi N, Safdar A, Keating MJ, Champlin RE, Aguilera EA, Tarrand JJ, Raad II. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore). 2006. 85:278–287.
22. van Kraaij MG, van Elden LJ, van Loon AM, Hendriksen KA, Laterveer L, Dekker AW, Nijhuis M. Frequent detection of respiratory viruses in adult recipients of stem cell transplants with the use of real-time polymerase chain reaction, compared with viral culture. Clin Infect Dis. 2005. 40:662–669.
23. Martino R, Ramila E, Rabella N, Munoz JM, Peyret M, Portos JM, Laborda R, Sierra J. Respiratory virus infections in adults with hematologic malignancies: a prospective study. Clin Infect Dis. 2003. 36:1–8.
24. Sung L, Alonzo TA, Gerbing RB, Aplenc R, Lange BJ, Woods WG, Feusner J, Franklin J, Patterson MJ, Gamis AS. Respiratory syncytial virus infections in children with acute myeloid leukemia: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2008. 51:784–786.
25. Marcolini JA, Malik S, Suki D, Whimbey E, Bodey GP. Respiratory disease due to parainfluenza virus in adult leukemia patients. Eur J Clin Microbiol Infect Dis. 2003. 22:79–84.
26. Khanna N, Steffen I, Studt JD, Schreiber A, Lehmann T, Weisser M, Fluckiger U, Gratwohl A, Halter J, Hirsch HH. Outcome of influenza infections in outpatients after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2009. 11:100–105.
27. Monto AS. Antivirals and influenza: frequency of resistance. Pediatr Infect Dis J. 2008. 27:S110–S112.
28. Boeckh M, Englund J, Li Y, Miller C, Cross A, Fernandez H, Kuypers J, Kim H, Gnann J, Whitley R. Randomized controlled multicenter trial of aerosolized ribavirin for respiratory syncytial virus upper respiratory tract infection in hematopoietic cell transplant recipients. Clin Infect Dis. 2007. 44:245–249.
29. Shima T, Yoshimoto G, Nonami A, Yoshida S, Kamezaki K, Iwasaki H, Takenaka K, Miyamoto T, Harada N, Teshima T, et al. Successful treatment of parainfluenza virus 3 pneumonia with oral ribavirin and methylprednisolone in a bone marrow transplant recipient. Int J Hematol. 2008. 88:336–340.
30. Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001. 98:573–578.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download