Abstract
Alveolar soft part sarcoma (ASPS) is a rare malignant soft-tissue neoplasm of unknown histogenesis. The two main sites of occurrence are the lower extremities in adults and the head and neck in children. We report the first case of pleural ASPS occurring in a 58-yr-old man who presented with progressive dyspnea. A computed tomographic scan of the thorax revealed a large enhancing pleural mass with pleural effusion in the left hemithorax. Wide excision of the pleural mass was performed. Histologically, the tumor consisted of organoid nests of large polygonal cells, the cytoplasm of which had eosinophilic and D-PAS positive granules. Immunohistochemical staining showed that the tumor cell nuclei were positive for transcription factor 3 (TFE3). The pleural ASPS with multiple bone metastases recurred 1 yr after surgery and the patient died of acute pulmonary embolism 1.5 yr after diagnosis.
Alveolar soft part sarcoma (ASPS) is a rare malignant soft-tissue neoplasm of unknown histogenesis, typically occurring in young patients. ASPS occurs in the deep soft tissues, most often in the buttock or thigh, with a smaller number of cases in other soft tissue locations such as the arm, chest, or retroperitoneum. In children, a substantial percentage of cases occur in the head and neck, often in the orbit or tongue. Morphologically, ASPS is characterized by uniform, organoid nests of polygonal tumor cells, separated by fibrovascular septa and delicate capillary-sized vascular channels. These nests display prominent cellular dyscohesion, leading to the distinctive pseudoalveolar pattern for which it is named. Histochemical staining is useful for establishing the diagnosis. Periodic acid-Schiff (PAS) preparation reveals varying amounts of intracellular glycogen and characteristically PAS-positive, diastase-resistant rhomboid or rod-shaped crystals (1). These crystals can also be identified by electron microscopy (EM). The PAS positive material is invariably present in these tumors, but determination of the crystalline form varies with the skill or luck of the observer (2). In previous studies, these structures were identified in as many as 80% to as few as 22% of cases (1-4) . Lieberman et al. (2) reported that these characteristic crystals were revealed by EM in 46% of the cases. Although the characteristic crystals are not always identified (1-4), PAS-positive granules or electron-dense granules, probably precursors of the crystals, appear to be important diagnostic feature of ASPS (5). Immunohistochemical findings can help to differentiate between ASPS and other diseases with similar histologic features. The differential diagnoses include metastatic renal cell carcinoma, paraganglioma, or granular cell tumor.
To date, five case reports of pulmonary ASPS without evidence of soft tissue tumor have been published in the literature (6-10), with one case arising from a pulmonary vein (10). However, primary pleural involvement of ASPS has not been reported since Christopherson et al. (11) first described and named it in 1952. We report a case of pleural ASPS with malignant pleural effusion and lung metastasis occurring in a 58-yr-old man. To the best of our knowledge, this is the first report on pleural ASPS with malignant pleural effusion.
A 58-yr-old man presented with progressive dyspnea on July 1, 2009. He also complained of cough and whitish sputum. He was an ex-smoker. Twenty years previously, a right nephrectomy had been performed at another hospital because of a right renal mass. The final pathologic findings were consistent with typical low-grade clear cell renal cell carcinoma (T1N0M0). A chest radiograph obtained upon admission showed a large pleural effusion in the left lung (Fig. 1A). Computed tomography (CT) images of the thorax showed a large enhancing pleural mass with pleural effusion in the left hemithorax, a 1.6 × 1.3 cm pulmonary nodule in the left upper lobe (LUL), and a right lower pleural nodule (Fig. 1B-D). Positron emission tomography-computed tomography (PET-CT) images showed a large left pleural effusion with heterogeneous hypermetabolic activity, mild-to-moderate hypermetabolic activity in the left lower paratracheal area, and a focal hypermetabolic nodular lesion in the LUL. No extrapulmonary lesions were seen on the PET-CT images (Fig. 2).
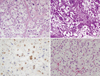
Although both cytologic and pathologic study of the pleural effusion and tissues obtained by diagnostic thoracentesis and pleural biopsy revealed atypical hyperchromatic and cytopathic cells, these findings were not sufficient for a definitive diagnosis. Thus, we performed excisional biopsy of the pleural mass by video-assisted thoracoscopic surgery (VATS). Because the biopsy result was suspicious for ASPS, we decided on open thoracotomy for a wide excision of the pleural mass with wedge resection of the pulmonary nodule in the LUL. Gross examination of the left pleural mass revealed a firm, 18.0 × 16.0 × 5.0 cm bilobulated, gray-white tumor with focal areas of necrosis and hemorrhage. Gross examination of the lung nodule showed a fragment of soft tissue that measured 1.5 × 1.2 cm. Pathologic examination of both pleural and lung specimens revealed an organoid or nested arrangement of tumor cells resulting in a pseudoalveolar pattern. The tumor cells were separated by fibrovascular septa and delicate capillary-sized vascular channels. The individual cells were large, rounded, or polygonal with one or two large nuclei and prominent nucleoli. The cytoplasm was abundant and appeared granular and sometimes vacuolated (Fig. 3A). Under Periodic acid Schiff staining with diastase treatment (D-PAS), abundant PAS-positive granules appeared in the tumor cells (Fig. 3B). The immunohistochemistry results were positive for TFE3 (Fig. 3C) and vimentin; equivocal for CD56, chromogranin, synaptophysin, and WT-1; and negative for epithelial membrane antigen (EMA), cytokeratin (CK), CK5/6, CK7, CK20, CD10, CD30, CD34, CD68, calretinin, CEA, smooth muscle actin (SMA), desmin, HMB-45, and S-100 protein.
Because it was necessary to rule out the metastasis of renal cell carcinoma to the pleura, which can generate similar pathologic findings to those of ASPS, we borrowed a slide from another hospital to review the pathologic findings of a renal tumor that was resected 20 yr previously and diagnosed as a low-grade clear cell renal cell carcinoma, and to compare the pathologic findings with those of a pleural tumor diagnosed as ASPS. The renal tumor cells had clear cytoplasms, distinct cell borders, small nuclei, and at low magnification, barely visible nucleoli. They were arranged in small solid nests (Fig. 3D). These pathologic findings are typical of a low-grade clear cell renal cell carcinoma and are clearly different from our pathologic finding of ASPS.
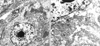
Electron microscopy showed that the cells contained numerous mitochondria, prominent rough endoplasmic reticulum, well-developed Golgi complexes, and many membrane-bound electron-dense granules (Fig. 4).
We recommended adjuvant chemotherapy after wide resection of the pleural tumor and metastasectomy of the lung nodule. However, the patient refused further treatment. The pleural ASPS with multiple bone metastases recurred 1 yr after surgery. The patient died of acute pulmonary embolism 1.5 yr after diagnosis.
We report a unique case of pleural ASPS with malignant pleural effusion and lung metastasis in a 58-yr-old man. Radiologic studies revealed a large pleural mass with pleural effusion and a small pulmonary nodule without any evidence of a soft tissue tumor elsewhere at the time of diagnosis. These findings suggest that the ASPS arose from the pleura and metastasized to the lung.
Although both D-PAS and EM are useful for a definitive diagnosis when they reveal characteristic crystalline materials, these are not always identified, as in our case. Instead, PAS-positive granules and electron-dense granules, both of which were identified in our case and were probably precursors of the crystals, can be identified even in crystal-deficient ASPS (5).
Renal cell carcinoma, whether primary or metastatic, often bears a striking resemblance to ASPS, but in most cases it can be distinguished by the absence of the characteristic PAS-positive crystalline material. Immunoreactivity for EMA is useful for a definitive diagnosis of renal cell carcinoma, as this antigen is absent in ASPS (1). Staining for TFE3 is also helpful, although it must be kept in mind that some pediatric renal cell carcinomas and granular cell tumors also express this antigen (12). In our patient, who had undergone right nephrectomy for renal cell carcinoma (T1N0M0, low-grade clear cell carcinoma), it was necessary to rule out metastasis of renal cell carcinoma to the pleura on the basis of clinical and pathologic findings. At the time of the diagnosis of ASPS, the patient was 58 yr of age; 20 yr had passed since the right nephrectomy had been performed to treat the renal cell carcinoma, and there was no evidence of a primary mass, including a left renal tumor. Moreover, the large pleural mass was located in the left hemithorax on the opposite side to the original renal cell carcinoma. The immunopathologic features clearly differed between the previous renal cell carcinoma and the present pleural ASPS, as did the immunohistochemical staining (negative for EMA and cytokeratin, positive for TFE3 and vimentin) of the tumor cells of the pleural mass (Fig. 3). We suggest that these features clearly discriminate pleural ASPS from pleural metastasis of renal cell carcinoma in adults. Glycogen is present in both ASPS and renal cell carcinoma, but is absent in granular cell tumor and paraganglioma. It is also noteworthy that the cells of granular cell tumors show strong S-100 protein staining (1). Paragangliomas, unlike ASPS, show strong expression of chromogranin A, synaptophysin and the presence of S-100 protein-positive sustentacular cells (1). Oncocytoma shows immunoreactivity to cytokerarin and vimentin but not to smooth muscle actin. Sugar tumors of the lung show strong immunoreactivity with the HMB-45 and Melan-A antibody. Adrenal cortical carcinomas and hepatocellular carcinomas may mimic ASPS by virtue of their abundant eosinophilic and clear cytoplasm. Immunohistochemical evidence of strong cytokeratin expression, expression of site-associated markers (e.g., Melan-A cross-reactivity in adrenal cortical carcinoma and HepPar1 in hepatocellular carcinoma), and the absence of TFE3 expression should help in this differential diagnosis (13). Malignant melanoma may simulate ASPS, but can be easily distinguished immunohistochemically by the presence of S100 protein, HMB45, and Melan-A (13). Alveolar rhabdomyosarcoma, despite its somewhat similar name, appears as an entirely different "small blue round cell tumor," which strongly expresses desmin and myogenin nuclear regulatory proteins (MyoD1). Perivascular epithelioid cell neoplasms, such as epithelioid angiomyolipoma, co-express smooth-muscle actin and melanocytic markers, and are almost always TFE3-negative.
It was recently discovered that ASPSs are characterized by a tumor-specific der(17)t(X;17) (p11;q25) translocation, which fuses the TFE3 gene at Xp11 to the ASPL gene at 17q25, creating an ASPL-TFE3 fusion protein (14). However, we could not perform the specific genetic study because our patient did not give his consent. An antibody directed against the C-terminus of the TFE3 has also emerged as a highly sensitive and specific marker of ASPS (12). Although TFE3 seems to be almost universally expressed in normal tissues, this expression is at very low levels. Strong nuclear expression of TFE3 is seen almost exclusively in tumors known to contain TFE3 gene fusions, such as ASPSs and rare pediatric renal carcinomas (12). It must be emphasized that only nuclear expression of TFE3 is of diagnostic value, as cytoplasmic staining (possibly non-specific) is seen in various tumors.
The ultimate prognosis of patients with ASPL is poor, despite the relatively slow growth of the tumor. The most important prognostic factors are the age at diagnosis (younger patients have a better prognosis), size of the tumor (larger tumors have a worse prognosis), and the presence of metastatic disease at presentation (2). The prevalence of metastasis upon presentation increases with the patient's age at the time of diagnosis (2).
Treatment is not particularly promising, with radical resection being the therapy of choice (2, 15). Most reviewers recommend radical surgical excision of primary and metastatic lesions combined with radiotherapy or chemotherapy (or both), although most have reported limited success with systemic therapies (16).
In conclusion, we encountered a case of ASPS involving the pleura and lung without any evidence of a soft tissue tumor elsewhere at the time of the initial diagnosis. We suggest this to be a case of primary ASPS of the pleura with malignant pleural effusion and lung metastasis. To the best of our knowledge, this is the first report of such a case to appear in the literature.
Figures and Tables
Fig. 1
Chest X-ray and chest computed tomography (CT) at admission. (A) Chest X-ray showing a large pleural effusion in the left hemithorax. (B-D) Chest CT, showing a huge pleural mass with pleural effusion in the left hemithorax (B), a pulmonary nodule in the left upper lobe (C, arrow), and a right pleural nodule (D, arrow).
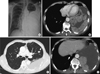
Fig. 2
Positron emission tomography-computed tomography (PET-CT) scans. (A) A focal hypermetabolic nodular lesion is seen in the left upper lobe. (B) A large pleural effusion with heterogeneous minimal-to-mild hypermetabolic activity is seen in the left lower lobe. (C) Minimal hypermetabolic nodular lesion is seen in right lower pleural surface (arrow). No other hyperrmetabolic lesions suggesting a primary tumor are seen in this PET-CT image.

Fig. 3
Light microscopy features of the resected pleural and renal tumors. The renal tumor was resected in another hospital 20 yr previously and diagnosed as a low-grade clear cell renal cell carcinoma. (A) The pleural tumor shows an organoid or nesting arrangement of cells with a pseudoalveolar pattern. The tumor cells are separated by fibrovascular septa and delicate capillary-sized vascular channels (H&E, × 200). (B) The cytoplasms of the tumor cells are D-PAS positive (D-PAS, × 200). (C) Immunohistochemistry of the pleural tumor. The nuclei are positive for TFE3 (TFE3, × 400). (D) The tumor cells of right renal mass have clear cytoplasms, distinct cell borders, and small nuclei. The cells are arranged in small solid nests (H&E, × 200).
References
1. Weiss SW, Goldblum JR. Malignant soft tissue tumors of uncertain type. Enzinger and Weiss' soft tissue tumors. 2008. 5th ed. Mosby Elsevier;1182–1191.
2. Lieberman PH, Brennan MF, Kimmel M, Erlandson RA, Garin-Chesa P, Flehinger BY. Alveolar soft-part sarcoma. A clinico-pathologic study of half a century. Cancer. 1989. 63:1–13.
3. Shipkey FH, Lieberman PH, Foote FW Jr, Stewart FW. Ultrastructure of alveolar soft part sarcoma. Cancer. 1964. 17:821–830.
4. Font RL, Jurco S 3rd, Zimmerman LE. Alveolar soft-part sarcoma of the orbit: a clinicopathologic analysis of seventeen cases and a review of the literature. Hum Pathol. 1982. 13:569–579.
5. Tucker JA. Crystal-deficient alveolar soft part sarcoma. Ultrastruct Pathol. 1993. 17:279–286.
6. Kim YD, Lee CH, Lee MK, Jeong YJ, Kim JY, Park do Y, Sol MY. Primary alveolar soft part sarcoma of the lung. J Korean Med Sci. 2007. 22:369–372.
7. Devisme L, Mensier E, Bisiau S, Bloget F, Gosselin B. Alveolar sarcoma. Report of a case. Ann Pathol. 1996. 16:49–52.
8. Kim NR, Lee MS, Yoon YC, Kim DS, Lee KS, Suh GY, Kim J, Han JH. Alveolar soft part sarcoma of the lung: a report of six cases and clinicopathological analysis. Korean J Pathol. 2003. 37:87–92.
9. Trabelsi A, Ben Abdelkrim S, Taher Yacoubi M, Mlika S, Hmissa S, Mokni M, Korbi S. Primary alveolar soft part sarcoma of the lung. Rev Mal Respir. 2009. 26:329–332.
10. Tsutsumi Y, Deng YL. Alveolar soft part sarcoma of the pulmonary vein. Acta Pathol Jpn. 1991. 41:771–777.
11. Christopherson WM, Foote FW, Stewart FW. Alveolar soft-part sarcomas. Structurally characteristic tumors of uncertain histogenesis. Cancer. 1952. 5:100–111.
12. Argani P, Lal P, Hutchinson B, Lui MY, Reuter VE, Ladanyi M. Aberrant nuclear immunoreactivity for TFE3 in neoplasms with TFE3 gene fusions: a sensitive and specific immunohistochemical assay. Am J Surg Pathol. 2003. 27:750–761.
13. Folpe AL, Deyrup AT. Alveolar soft-part sarcoma: a review and update. J Clin Pathol. 2006. 59:1127–1132.
14. Ladanyi M, Lui MY, Antonescu CR, Krause-Boehm A, Meindl A, Argani P, Healey JH, Ueda T, Yoshikawa H, Meloni-Ehrig A, et al. The der(17) t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene. 2001. 20:48–57.
15. Zarrin-Khameh N, Kaye KS. Alveolar soft part sarcoma. Arch Pathol Lab Med. 2007. 131:488–491.
16. Reichardt P, Lindner T, Pink D, Thuss-Patience PC, Kretzschmar A, Dorken B. Chemotherapy in alveolar soft part sarcoma. What do we know? Eur J Cancer. 2003. 39:1511–1516.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download