1. Gossec L, Dougados M, Goupille P, Cantagrel A, Sibilia J, Meyer O, Sany J, Daurès JP, Combe B. Prognostic factors for remission in early rheumatoid arthritis: a multiparameter prospective study. Ann Rheum Dis. 2004. 63:675–680.
2. Alenfeld FE, Diessel E, Brezger M, Sieper J, Felsenberg D, Braun J. Detailed analyses of periarticular osteoporosis in rheumatoid arthritis. Osteoporos Int. 2000. 11:400–407.
3. Crewson PE. Reader agreement studies. AJR Am J Roentgenol. 2005. 184:1391–1397.
4. Ahn IE, Ju JH, Park SH, Kim HY. Radiologic observation: repair of focal bone erosions after humanized antitumor necrosis factor antibody adalimumab therapy in a patient with rheumatoid arthritis. Clin Rheumatol. 2010. 29:211–213.
5. Hoff M, Kvien TK, Kälvesten J, Elden A, Haugeberg G. Adalimumab therapy reduces hand bone loss in early rheumatoid arthritis: explorative analyses from the PREMIER study. Ann Rheum Dis. 2009. 68:1171–1176.
6. Smolen JS, Han C, Bala M, Maini RN, Kalden JR, van der Heijde D, Breedveld FC, Furst DE, Lipsky PE. Evidence of radiographic benefit of treatment with infliximab plus methotrexate in rheumatoid arthritis patients who had no clinical improvement: a detailed subanalysis of data from the anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study. Arthritis Rheum. 2005. 52:1020–1030.
7. Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, Singh A, Pedersen RD, Koenig AS, Freundlich B. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008. 372:375–382.
8. St Clair EW, van der Heijde DM, Smolen JS, Maini RN, Bathon JM, Emery P, Keystone E, Schiff M, Kalden JR, Wang B, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004. 50:3432–3443.
9. Hoff M, Haugeberg G, Kvien TK. Hand bone loss as an outcome measure in established rheumatoid arthritis: 2-year observational study comparing cortical and total bone loss. Arthritis Res Ther. 2007. 9:R81.
10. van der Heijde DM. Plain X-rays in rheumatoid arthritis: overview of scoring methods, their reliability and applicability. Baillieres Clin Rheumatol. 1996. 10:435–453.
11. Deodhar AA, Brabyn J, Jones PW, Davis MJ, Woolf AD. Measurement of hand bone mineral content by dual energy x-ray absorptiometry: development of the method, and its application in normal volunteers and in patients with rheumatoid arthritis. Ann Rheum Dis. 1994. 53:685–690.
12. Jensen T, Klarlund M, Hansen M, Jensen KE, Podenphant J, Hansen TM, Skjødt H, Hyldstrup L. Bone loss in unclassified polyarthritis and early rheumatoid arthritis is better detected by digital x ray radiogrammetry than dual x ray absorptiometry: relationship with disease activity and radiographic outcome. Ann Rheum Dis. 2004. 63:15–22.
13. Böttcher J, Pfeil A. Diagnosis of periarticular osteoporosis in rheumatoid arthritis using digital X-ray radiogrammetry. Arthritis Res Ther. 2008. 10:103.
14. Peel NF, Spittlehouse AJ, Bax DE, Eastell R. Bone mineral density of the hand in rheumatoid arthritis. Arthritis Rheum. 1994. 37:983–991.
15. Haugeberg G, Lodder MC, Lems WF, Uhlig T, Ørstavik RE, Dijkmans BA, Kvien TK, Woolf AD. Hand cortical bone mass and its associations with radiographic joint damage and fractures in 50-70 year old female patients with rheumatoid arthritis: cross sectional Oslo-Truro-Amsterdam (OSTRA) collaborative study. Ann Rheum Dis. 2004. 63:1331–1334.
16. Haugeberg G, Green MJ, Conaghan PG, Quinn M, Wakefield R, Proudman SM, Stewart S, Hensor E, Emery P. Hand bone densitometry: a more sensitive standard for the assessment of early bone damage in rheumatoid arthritis. Ann Rheum Dis. 2007. 66:1513–1517.
17. Alves C, Colin EM, van Oort WJ, Sluimer JP, Hazes JM, Luime JJ. Periarticular osteoporosis: a useful feature in the diagnosis of early rheumatoid arthritis? Reliability and validity in a cross-sectional diagnostic study using dual-energy X-ray absorptiometry. Rheumatology (Oxford). 2011. 50:2257–2263.
18. Deodhar AA, Brabyn J, Pande I, Scott DL, Woolf AD. Hand bone densitometry in rheumatoid arthritis, a five year longitudinal study: an outcome measure and a prognostic marker. Ann Rheum Dis. 2003. 62:767–770.
19. Güler-Yüksel M, Allaart CF, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, van Groenendael JH, Mallée C, de Bois MH, Breedveld FC, Dijkmans BA, Lems WF. Changes in hand and generalised bone mineral density in patients with recent-onset rheumatoid arthritis. Ann Rheum Dis. 2009. 68:330–336.
20. Kaya A, Ozgocmen S, Ardicoglu O, Kamanli A, Gudul H. Relationship between grip strength and hand bone mineral density in healthy adults. Arch Med Res. 2005. 36:603–606.
21. Vehmas T, Solovieva S, Riihimäki H, Luoma K, Leino-Arjas P. Hand workload and the metacarpal cortical index. A study of middle-aged teachers and dentists. Osteoporos Int. 2005. 16:672–680.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


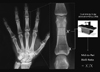





 XML Download
XML Download