Abstract
Several studies have reported that ABO blood group, hepatitis B virus (HBV) and hepatitis C virus (HCV) infection contribute to the development of pancreatic cancer. The aim of this study was to evaluate the association between these factors and pancreatic cancer in the Korean population. We retrospectively recruited 753 patients with pancreatic cancer and 3,012 healthy controls, matched 4 to 1 with cancer patients for age and sex, between 2001 and 2011, at the National Cancer Center, Korea. A multivariate logistic regression analysis was employed to estimate adjusted odds ratios (AORs). The AOR for pancreatic cancer in subjects with non-O blood types (A, AB, and B), compared to blood type O, was 1.29 (95% CI, 1.05-1.58; P = 0.01). Seropositivity for hepatitis B virus surface antigen was not significantly related to pancreatic cancer, either in univariate (odds ratio 1.03; 95% CI, 0.69-1.53; P = 0.91) or multivariate analysis (AOR, 1.02; 95% CI, 0.67-1.56; P = 0.93). The AOR for pancreatic cancer in subjects displaying seropositivity for anti-HCV was 2.30 (95% CI, 1.30-4.08; P < 0.01). Our results suggest that the non-O blood types and anti-HCV seropositivity, but not HBV infection, may increase the risk of developing pancreatic cancer in Korea, where HBV is endemic.
Pancreatic cancer is estimated as the ninth most frequent cancer and fifth most common cause of cancer-related death in Korea (1). Ductal adenocarcinoma accounts for 85% to 90% of pancreatic tumors (2). Pancreatic cancer is rarely diagnosed before 45 yr of age, but its occurrence rises sharply thereafter. The incidence of pancreatic cancer is higher in men than women, and in Africans, compared with the Caucasian population (3). Prognosis for patients with this disease is extremely poor, with a 7.6% 5-yr survival rate in Korea (1), mainly due to unresectable disease in 80%-90% of patients at the time of diagnosis (4). Pancreatic cancer patients seldom exhibit disease-specific symptoms until late in the course of disease progression, and the impact of standard therapy is thus limited.
While the etiology of pancreatic cancer remains to be established, several known genetic and environmental factors are associated with its development. So far, risk factors accounting for up to 30% of the disease have been established (5). Among the few risk factors identified to date, cigarette smoking is the most consistent (6). Current smokers are at approximately double the risk as non-smokers, with a trend towards increasing risk according to the frequency or duration of smoking exposure (5). Inconsistencies in the patterns of cigarette smoking and incidence between different countries, as well as the low relative risk, suggest that the disease is only partly attributable to smoking (up to 20%), and therefore, other risk factors are likely to be important. Diabetes mellitus (7) and chronic pancreatitis (8) are additional predisposing factors of the disease. However, diabetes as a result of pancreatic cancer development is not infrequent, and chronic pancreatitis explains only less than 3% of pancreatic cancer cases. An association with obesity has been reported (9), but the effects of dietary factors and physical activity are currently unclear. Genetic susceptibility plays a role, with some cases being familial or related to hereditary melanoma, Peutz-Jeghers syndrome, hereditary breast or ovarian cancer, familial pancreatitis, or hereditary nonpolyposis colon cancer (10). Hereditary factors, such as germline mutations, account for only about 10% of the total burden of pancreatic cancer (11).
Recent research has reawakened interest in several additional factors. Earlier studies have reported a higher association of pancreatic cancer with the non-O blood groups, compared to O (12-16). A report based on a large, prospective study involving almost one million subjects with years of follow-up showed a link between ABO blood type and pancreatic cancer (13). In agreement with several previously published smaller-scale studies, these investigators showed that non-O blood types accounted for 17% of all new pancreatic cancers. Other recent studies have additionally detected a link between hepatitis B virus (HBV) or hepatitis C virus (HCV) infection and pancreatic cancer, indicating a stronger relationship with HBV than HCV (17, 18). However, the findings to date are inconsistent, and the exact mechanism linking pancreatic cancer and blood groups is currently unclear. Therefore, further investigation of these risk factors is warranted to determine their significance in pancreatic cancer.
The principal aim of this study was to evaluate the possible association between ABO blood type and pancreatic cancer in Korea. We further assessed whether HBV and HCV infections are associated with pancreatic cancer risk.
In total, 753 patients with pancreatic cancer and 3,012 healthy controls, matched 4 to 1 with pancreatic cancer patients for age and sex, were retrospectively recruited at the National Cancer Center, Korea, between 2001 and 2011. Our subjects included patients with newly diagnosed pancreatic adenocarcinoma who were evaluated and treated at the National Cancer Center, Korea. Inclusion criteria included clinical or pathological diagnosis of pancreatic ductal adenocarcinoma. Clinical diagnosis was based on typical radiologic and clinical findings. Causes of death were ascertained by linking the study patients with the National Death Certificate Database of Korea. Exclusion criteria were presence of other types of pancreatic neoplasm, such as neuroendocrine tumor or intraductal mucinous neoplasm of pancreas. Among the 2,243 eligible pancreatic cancer patients in the hospital database, 753 (34%) had a historical or current ABO blood group available, of which 625 (83%) cases were pathologically confirmed adenocarcinoma. Data on sex, age at diagnosis of cancer, follow-up time after diagnosis, and tumor staging were collected from electronic medical records.
Eligible control subjects were selected from individuals in the Cancer Screening Cohort, who were subjected to routine health examinations at the Center for Cancer Prevention and Detection of National Cancer Center, Korea, between 2001 and 2011. In total, 3,012 healthy subjects were included. Control patients were randomly selected from these subjects and matched 4:1 with pancreatic cancer patients by age, sex, and date of admission or visit. Case and control frequencies were matched by age (± 5 yr) and sex. As part of the health maintenance examination, controls underwent laboratory tests, including tests for HBsAg, anti-Hbs antibody, anti-HCV antibody, ABO blood type, chest radiography, upper endoscopy, occult blood in stool, and abdominal sonography and/or contrast-enhanced computed tomography (CT). Women were additionally subjected to mammography and cervical smear.
We retrospectively reviewed data, including age, sex, use of tobacco, and diabetes, but were unable to obtain reliable information on pre-diagnosis body mass index, personal or family history of cancer, and alcohol use. The chemiluminescent microparticle immunoassay (Abbott Laboratories, Chicago, IL, USA) was used as a screening test for the presence of HBsAg in blood. The presence of antibodies to HBsAg (anti-HBs) and HCV (anti-HCV) was detected using a chemiluminescent immunoassay (Siemens Healthcare Diagnostics, Chicago, IL, USA). Chronic HBV and HCV infection were defined as the presence of HBsAg and anti-HCV antibodies, respectively.
The demographic characteristics and proportions of potential risk factors (including smoking and diabetes) were compared among patients and controls. The t-test was used to compare means of continuous variables between patients and controls, and the chi-square test applied to compare proportions. Multivariate logistic regression analyses, including age, gender, smoking history and diabetes, were performed to estimate the adjusted odds ratios (AOR). For each factor, we calculated the AOR and 95% confidence interval (CI) using maximum likelihood estimation. Overall survival (OS) was defined as the time from initiation of treatment to the date of death. OS were assessed by the Kaplan-Meier method, and 95% CI for the median time to an event were calculated.
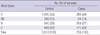
The mean age (± standard deviation) of pancreatic cancer patients was 62.3 ± 9.7 yr and 59.7 ± 7.5 yr for controls. The majority of study subjects were male (62.1%). Table 1 presents the ABO blood group distribution of 753 pancreatic cancer patients, compared with ABO groups of the 3,012 healthy controls. The frequency distribution patterns of ABO blood groups of controls in the current study were similar to those of Korean blood donors reported in the Korea Health and Welfare White Paper (1995).
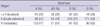
Compared with blood type O, the odds ratios (95% CI) in univariate analysis were 1.25 (1.04-1.53; P = 0.02) for non-O (A, B, or AB) groups after adjusting for age and gender (Table 2). AOR for pancreatic cancer in subjects with non-O blood types was 1.29 (95% CI, 1.05-1.58; P = 0.01). The odds ratios in univariate analysis were determined as 1.36 (1.10-1.68; P = 0.03) for blood type A, 1.21 (0.91-1.60; P = 0.76) for type AB, and 1.15 (0.92-1.44; P = 0.79) for type B, compared with blood type O, after adjusting for age and gender. The AORs for pancreatic cancer in subjects with blood types A, AB, and B were 1.36 (1.09-1.71; P = 0.08), 1.29 (0.96-1.74; P = 0.47), and 1.20 (0.94-1.52; P = 0.94), respectively.
Seropositivity for HBsAg was not significantly related to pancreatic cancer, either in univariate (Odds ratio 1.03; 95% CI, 0.69-1.53; P = 0.91) or multivariate analysis (AOR, 1.02; 95% CI, 0.67-1.56; P = 0.93) (Table 2). The AOR for pancreatic cancer in subjects with seropositivity for anti-HCV was 2.30 (95% CI, 1.30-4.08; P < 0.01). Other risk factors for pancreatic cancer included presence of diabetes (AOR, 2.70; 95% CI, 2.20-3.31; P < 0.01) and smoking (AOR, 1.47; 95% CI, 1.16-1.86; P < 0.01).
TNM stages of pancreatic cancer in the present study grouped by blood type are shown in Table 3. No significant differences in the TNM stages of tumors among patients with various blood groups were evident (P = 0.413). The median survival times in subjects with blood groups A, B, AB and O were 9.1, 9.6, 7.4, and 7.8 months, respectively, which were not significant, as indicated by the log-rank test (P = 0.106). In addition, we observed no marked differences in survival times between the non-O (A, B and AB) and O blood groups (P = 0.428).
Studies performed several decades ago initially suggested an association between blood type A and increased risk of pancreatic cancer, compared to blood groups O or B (19-21). However, increased pancreatic cancer risk in blood group B patients among 224 cases was observed, compared with a randomly selected group of patients admitted with nonmalignant diseases and blood donors in a more recent study (22). Results from two large, independent prospective cohorts of Caucasians from the United States suggested that compared with blood group O subjects, those with non-O blood types (A, AB or B) were more likely to develop pancreatic cancer (13). Similarly, in two large case-control studies on Chinese patients, blood group O was associated with a lower incidence of pancreatic cancer, compared with blood groups A and AB (16, 23). In the current investigation involving a large case-control Korean study, patients with blood group O had a lower incidence of pancreatic cancer, compared to those with non-O blood groups, consistent with previous reports. To our knowledge, this is the first study showing a correlation between blood group O and pancreatic cancer development in the Korean population.
ABO blood group antigens are widely distributed throughout the body in addition to their regular occurrence on the red blood cell surface. The ABO phenotype may be associated with risk of gastric cancer, gastric and duodenal ulcer, chronic atrophic gastritis, as well as pancreatic cancer (24). Human pancreatic cancer has been shown to express either A or B antigens corresponding to the individual blood group (25) or lose blood group antigen expression in 80% of the cases (26). Deletion of A, B, H or Lewis antigens and incompatible expression of A or B antigens has been reported as a cancer-associated event in the pancreas (27). Incompatible expression of blood group-related antigens is observed in pancreatic cancer cells, compared with patient blood group type, indicating that Lewis antigen expression in pancreatic cancer is independent of the blood group phenotype and may be useful as a tumor marker (28). A recent genome-wide association study revealed an association of a particular ABO locus on chromosome 9q34 with susceptibility to pancreatic cancer (29). This SNP maps to the first intron of the ABO blood type gene. However, limited information is currently available on the role of ABO blood group antigens in pancreatic cancer risk in Korea.
Recently, a retrospective analysis based on the surgical database at Washington University in St. Louis showed that ABO blood type does not affect overall survival among patients with resected pancreatic adenocarcinomas (30). However, among Chinese patients subjected to potentially curative operation, the median survival time of individuals with blood group O was significantly longer than that of those with non-O blood groups (16). The earlier study was limited to a population with resectable disease, accounting for only 10%-20% of patients with pancreatic cancer. Our data showed no association between ABO blood type and overall survival in patients with locally advanced and metastatic disease, as well as resected pancreatic cancer. This result suggested that ABO blood type is not associated with the risk for progression in pancreatic cancer.
The HBV antigen has been detected in the pancreatic acinar cells and pancreatic juice in patients with viral replication in the liver (31, 32). One hypothesis is that HBV can infect and replicate in human pancreatic cells and function in the oncogenesis of pancreatic carcinoma. However, it is not clear from a biologic viewpoint why resolved or occult HBV infection is associated with elevated pancreatic cancer risk. Previous epidemiological analyses have reported conflicting results on the relationship between chronic HBV infection and development of pancreatic cancer (17, 23, 33-35). Korea is one of the known endemic areas for chronic hepatitis B infection, and seropositivity of HBsAg was estimated as 7% to 9% in the 1980s, with about 60% to 70% of adults showing evidence of present or past HBV infection (36). Epidemiological findings based on the 1998 Korea National Health and Nutrition Survey disclosed HBsAg seropositivity of 5.1% in males and 4.1% in females aged 10 yr or over. This figure was similar among patients with pancreatic cancer and control subjects, resulting in AOR for HBsAg positivity of 1.02 (95% CI, 0.67-1.56; P=0.93). Further studies are required to confirm this finding, and the potential association between hepatitis B viral infection and pancreatic cancer, although interesting, should be interpreted with caution.
A retrospective cohort study reported that risk of pancreatic cancer is slightly elevated in patients with HCV, which is attenuated after adjusting for alcohol use, pancreatitis, and other variables (18). In the present case-control study, the AOR for pancreatic cancer in subjects with seropositivity for anti-HCV was 2.30 (95% CI, 1.30-4.08; P=0.004). However, the biological rationale explaining the association between HCV infection and pancreatic cancer is yet to be established. Serum levels of pancreatic enzymes have been shown to increase with liver disease progression in patients diagnosed with viral hepatitis (37, 38). However, we were limited in terms of our ability to confirm HCV infection based on the presence of serum HCV RNA. Serological assays designed to detect antibody to HCV are associated with a high degree of false positivity in regions with low prevalence and low-risk populations. We were additionally restricted in our ability to adjust for alcohol use, a potential risk factor for pancreatic cancer. These factors may be confounders of the association between HCV and pancreatic cancer. Interestingly, the risk of pancreatic cancer in patients with anti-HCV seropositivity was not attenuated after adjusting for smoking and diabetes, which are known risk factors for the disease. Additional epidemiological studies focusing on the association of HCV with pancreatic cancer are therefore necessary.
Our results collectively suggest that non-O blood types and seropositivity for anti-HCV, but not HBV infection, may increase the risk of developing pancreatic cancer in Korea, where HBV is endemic. Further research is necessary to define the mechanisms by which ABO blood type or closely linked genetic variants influence pancreatic cancer risk in Korea.
Figures and Tables
References
1. Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010. 25:1113–1121.
2. Cubilla AL, Fitzgerald PJ. Tumors of the exocrine pancreas. AFIP Atlas of tumor pathology. series 2. fascicle 19. 1984. Washington, D.C.: Armed Forces Institute of Pathology.
3. Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, Mariotto A, Feuer EJ, Edwards BK, editors. SEER cancer statistics review, 1975-2001. accessed on 1 April 2011. Bethesda: National Cancer Institute;http://seer.cancer.gov/csr/1975_2001.
4. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011. 378:607–620.
5. Risch HA. Pancreatic cancer: Helicobacter pylori colonization, N-nitrosamine exposures, and ABO blood group. Mol Carcinog. 2012. 51:109–118.
6. Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol. 2009. 6:699–708.
7. Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005. 92:2076–2083.
8. Lowenfels AB, Maisonneuve P, Lankisch PG. Chronic pancreatitis and other risk factors for pancreatic cancer. Gastroenterol Clin North Am. 1999. 28:673–685.
9. Berrington de Gonzalez A, Sweetland S, Spencer E. A meta-analysis of obesity and the risk of pancreatic cancer. Br J Cancer. 2003. 89:519–523.
10. Hansel DE, Kern SE, Hruban RH. Molecular pathogenesis of pancreatic cancer. Annu Rev Genomics Hum Genet. 2003. 4:237–256.
11. Lowenfels AB, Maisonneuve P. Pancreatic cancer: development of a unifying etiologic concept. Ann N Y Acad Sci. 1999. 880:191–200.
12. Risch HA, Yu H, Lu L, Kidd MS. ABO blood group, Helicobacter pylori seropositivity, and risk of pancreatic cancer: a case-control study. J Natl Cancer Inst. 2010. 102:502–505.
13. Wolpin BM, Chan AT, Hartge P, Chanock SJ, Kraft P, Hunter DJ, Giovannucci EL, Fuchs CS. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009. 101:424–431.
14. Wolpin BM, Kraft P, Gross M, Helzlsouer K, Bueno-de-Mesquita HB, Steplowski E, Stolzenberg-Solomon RZ, Arslan AA, Jacobs EJ, Lacroix A, et al. Pancreatic cancer risk and ABO blood group alleles: results from the pancreatic cancer cohort consortium. Cancer Res. 2010. 70:1015–1023.
15. Nakao M, Matsuo K, Hosono S, Ogata S, Ito H, Watanabe M, Mizuno N, Iida S, Sato S, Yatabe Y, et al. ABO blood group alleles and the risk of pancreatic cancer in a Japanese population. Cancer Sci. 2011. 102:1076–1080.
16. Ben Q, Wang K, Yuan Y, Li Z. Pancreatic cancer incidence and outcome in relation to ABO blood groups among Han Chinese patients: a case-control study. Int J Cancer. 2011. 128:1179–1186.
17. Hassan MM, Li D, El-Deeb AS, Wolff RA, Bondy ML, Davila M, Abbruzzese JL. Association between hepatitis B virus and pancreatic cancer. J Clin Oncol. 2008. 26:4557–4562.
18. El-Serag HB, Engels EA, Landgren O, Chiao E, Henderson L, Amaratunge HC, Giordano TP. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: a population-based study of U.S. veterans. Hepatology. 2009. 49:116–123.
19. Vogel F. Controversy in human genetics. ABO blood groups and disease. Am J Hum Genet. 1970. 22:464–475.
20. Newell GR, Gordon JE, Monlezun AP, Horwitz JS. ABO blood groups and cancer. J Natl Cancer Inst. 1974. 52:1425–1430.
21. Aird I, Lee DR, Roberts JA. ABO blood groups and cancer of oesophagus, cancer of pancreas, and pituitary adenoma. Br Med J. 1960. 1:1163–1166.
22. Annese V, Minervini M, Gabbrielli A, Gambassi G, Manna R. ABO blood groups and cancer of the pancreas. Int J Pancreatol. 1990. 6:81–88.
23. Wang DS, Chen DL, Ren C, Wang ZQ, Qiu MZ, Luo HY, Zhang DS, Wang FH, Li YH, Xu RH. ABO blood group, hepatitis B viral infection and risk of pancreatic cancer. Int J Cancer. 2012. 131:461–468.
24. Edgren G, Hjalgrim H, Rostgaard K, Norda R, Wikman A, Melbye M, Nyrén O. Risk of gastric cancer and peptic ulcers in relation to ABO blood type: a cohort study. Am J Epidemiol. 2010. 172:1280–1285.
25. Ernst C, Atkinson B, Wysocka M, Blaszczyk M, Herlyn M, Sears H, Steplewski Z, Koprowski H. Monoclonal antibody localization of Lewis antigens in fixed tissue. Lab Invest. 1984. 50:394–400.
26. Davidsohn I, Ni LY, Stejskal R. Tissue isoantigens A, B, and H in carcinoma of the pancreas. Cancer Res. 1971. 31:1244–1250.
27. Itzkowitz SH, Yuan M, Ferrell LD, Ratcliffe RM, Chung YS, Satake K, Umeyama K, Jones RT, Kim YS. Cancer-associated alterations of blood group antigen expression in the human pancreas. J Natl Cancer Inst. 1987. 79:425–434.
28. Pour PM, Tempero MM, Takasaki H, Uchida E, Takiyama Y, Burnett DA, Steplewski Z. Expression of blood group-related antigens ABH, Lewis A, Lewis B, Lewis X, Lewis Y, and CA 19-9 in pancreatic cancer cells in comparison with the patient's blood group type. Cancer Res. 1988. 48:5422–5426.
29. Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA, Bueno-de-Mesquita HB, Gross M, Helzlsouer K, Jacobs EJ, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. 2009. 41:986–990.
30. Dandona M, Gao F, Linehan DC, Wang-Gillam A. Re: ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2010. 102:135–137.
31. Shimoda T, Shikata T, Karasawa T, Tsukagoshi S, Yoshimura M, Sakurai I. Light microscopic localization of hepatitis B virus antigens in the human pancreas. Possibility of multiplication of hepatitis B virus in the human pancreas. Gastroenterology. 1981. 81:998–1005.
32. Hoefs JC, Renner IG, Askhcavai M, Redeker AG. Hepatitis B surface antigen in pancreatic and biliary secretions. Gastroenterology. 1980. 79:191–194.
33. Iloeje UH, Yang HI, Jen CL, Su J, Wang LY, You SL, Lu SN, Chen CJ. Risk of pancreatic cancer in chronic hepatitis B virus infection: data from the REVEAL-HBV cohort study. Liver Int. 2010. 30:423–429.
34. Zhu F, Li HR, Du GN, Chen JH, Cai SR. Chronic hepatitis B virus infection and pancreatic cancer: a case-control study in southern China. Asian Pac J Cancer Prev. 2011. 12:1405–1408.
35. Berrington de Gonzalez A, Yun JE, Lee SY, Klein AP, Jee SH. Pancreatic cancer and factors associated with the insulin resistance syndrome in the Korean cancer prevention study. Cancer Epidemiol Biomarkers Prev. 2008. 17:359–364.
36. Lee DH, Kim JH, Nam JJ, Kim HR, Shin HR. Epidemiological findings of hepatitis B infection based on 1998 National Health and Nutrition Survey in Korea. J Korean Med Sci. 2002. 17:457–462.
37. Katakura Y, Yotsuyanagi H, Hashizume K, Okuse C, Okuse N, Nishikawa K, Suzuki M, Iino S, Itoh F. Pancreatic involvement in chronic viral hepatitis. World J Gastroenterol. 2005. 11:3508–3513.
38. Yoffe B, Bagri AS, Tran T, Dural AT, Shtenberg KM, Khaoustov VI. Hyperlipasemia associated with hepatitis C virus. Dig Dis Sci. 2003. 48:1648–1653.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download