Abstract
To determine the approximate incidence and clinical features of pernicious anemia in a Korean population, we retrospectively analyzed clinical data for patients with pernicious anemia who were diagnosed between 1995 and 2010 at five hospitals in Chungnam province. Ninety-seven patients were enrolled, who accounted for 24% of patients with vitamin B12 deficiency anemia. The approximate annual incidence of pernicious anemia was 0.3 per 100,000. The median age was 66 (range, 32-98) yr, and the male/female ratio was 1.25. Anemia-associated discomfort was the most common symptom (79.4%), followed by gastrointestinal and neurological symptoms (78.4% and 38.1%, respectively). Pancytopenia was found in 36 patients (37.1%), and autoimmune disorders were found in 15 patients (15.5%). Antibody to intrinsic factor was detected in 62 (77.5%) of 80 patients examined, and antibody to parietal cells was detected in 35 (43.2%) of 81 patients examined. Of the 34 patients who underwent tests for Helicobacter pylori, 7 (12.5%) were positive. The anemia-associated and gastrointestinal symptoms resolved completely in all patients after intramuscular injection of cobalamin, whereas neurological symptoms remained in some. In conclusion, pernicious anemia is less frequent in Koreans than in Western populations; however, the clinical features of this disorder in Koreans do not differ from those of Western cases.
Pernicious anemia is a megaloblastic anemia caused by intrinsic factor deficiency secondary to autoimmune destruction of the acid- and pepsin-secreting portion of the gastric mucosa. Pernicious anemia is the most common cause of vitamin B12 deficiency and one of the most common autoimmune diseases in Western countries (1, 2). Pernicious anemia was previously thought to be a rare disease in East Asia, but is now regarded as a disorder affecting virtually all racial and ethnic groups (3). Although reports describing pernicious anemia in the East Asia are quite rare, there is some information on the clinical features of this disorder in Japan and China. For example, Sugihara and Yawata (4) reported the clinical statistical data of pernicious anemia in a Japanese population in 1992. Recently, Chan et al. (5) reported the clinical features of 181 patients with pernicious anemia and demonstrated that pernicious anemia is a major cause of megaloblastic anemia in Chinese individuals. In Korea, the first case of pernicious anemia was reported in 1966 (6). Since then, only case reports or small series have been reported (7-9). For example, Song et al. (8) described eight patients with pernicious anemia among 45 patients with vitamin B12 deficiency megaloblastic anemia, and Chun et al. (9) reported the clinical features of 22 patients with pernicious anemia. The incidence, prevalence, and clinical features of this disorder remain largely unknown in Korea. Thus, we performed a multicenter retrospective study to estimate the approximate incidence and define the clinical features of pernicious anemia in a Korean population.
We retrospectively analyzed the medical records of patients who were newly diagnosed with pernicious anemia between 1995 and 2010 at five university hospitals in Chungnam province. The diagnosis of pernicious anemia was made when serum vitamin B12 levels were < 200 pg/mL in patients with anemia (hemoglobin level of < 13.0 g/dL in men and < 12.0 g/dL in women) who displayed one of the following abnormalities: positive anti-intrinsic factor antibody, positive anti-parietal cell antibody with moderate to severe atrophic gastritis of the stomach body without Helicobacter pylori infection, histologically confirmed severe atrophic gastritis of the stomach body, or the presence of other autoimmune disorders.
We collected and analyzed the results of clinical presentations, complete blood counts (CBC), peripheral blood (PB) smear morphology, chemistry, bone marrow (BM) studies, endoscopy and biopsy, anti-intrinsic factor antibody, anti-parietal cell antibody, the presence of autoimmune disorders, and response to cobalamin replacement therapy. In most cases, serum vitamin B12 levels were measured by eletrochemiluminescence immunoassay. Anti-intrinsic factor antibody and anti-parietal cell antibody were measured by immunoradioassay and indirect immunofluorescence, respectively. Responses to cobalamin replacement therapy were assessed by changes in the CBC and resolution of symptoms after 3 months of treatment.
Between January 1, 1995, and December 31, 2010, a total of 416 patients was diagnosed with megaloblastic anemia. Among these patients, 405 and 11 were associated with vitamin B12 deficiency and folate deficiency, respectively. Among the 405 patients with vitamin B12 deficiency, 97 (23%) were diagnosed with pernicious anemia, and the cause in 243 (60.0%) was determined to be gastrectomy. The remaining 65 patients were not fully evaluated for etiology (Table 1). Most cases of pernicious anemia were diagnosed after 2000, and only four (4.1%) cases were diagnosed before 2000. The median age of the 97 patients (54 men and 43 women) at diagnosis was 66 yr (range, 32-98 yr) (Table 2). The years in which patient recruitment began differed among participating hospitals, but all hospitals enrolled patients who were diagnosed between 2005 and 2010 (61 patients in total). If all newly developed cases of pernicious anemia in Chungnam province were seen by the five hospitals between 2005 and 2010, the approximate annual incidence of pernicious anemia would be 0.3 per 100,000 during this period (Fig. 1).
The presenting complaints included generalized weakness (66.0 %), sore tongue (53.6%), anorexia (42.3%), numbness (37.1%), exertional dyspnea (36.1%), vertigo (33.0%), involuntary weight loss of > 4.5 kg or > 5% of one's body weight over the period of 6 months (20.6%), forgetfulness (4.1%), diarrhea (2.0%), and jaundice (1.0%). Anemia-associated discomfort was the most common symptom (79.4%); followed by gastrointestinal symptoms that included anorexia, sore tongue, and weight loss (78.4 %); and neurological symptoms (38.1%). The median duration of symptoms at diagnosis was 3 months (range, 1-144 months) (Table 2).
At presentation, Hashimoto's thyroiditis was found in six (6.1%) patients, vitiligo in three (3.0%), and Graves' disease in four (4.1 %). Type 1 diabetes mellitus, Addison's disease, and ankylosing spondylitis were found in one patient each. Two (2.0%) patients already had stomach cancer at the time of diagnosis of pernicious anemia (Table 2).
CBC at presentation revealed anemia with a hemoglobin of < 7.0 g/dL in 46 (47.2%) patients and between 7.0 and 10.0 g/dL in 35 (36.1%) patients. The median hemoglobin level was 7.3 g/dL (range, 3.1-12.9 g/dL). White blood cell (WBC) counts were < 4 × 109/L in 44 (45.4%) patients. The median white blood cell count was 4.14 × 109/L (range, 1.4-12.58 × 109/L). Platelets counts were < 50 × 109/L in 12 (12.4%) patients, 50 to 150 × 109/L in 51 (52.6%) patients, and > 150 × 109/L in 34 (35.1%) patients. The median platelet count was 113 × 109/L (range, 23-373 × 109/L). Pancytopenia was observed in 36 (37.1%) patients. Macrocytosis with a mean corpuscular volume of > 99 fL was noticed in 86 (88.7%) patients (median, 118; range, 84-144 fL). Hypersegmented neutrophils, defined as the presence of ≥ 5% of five-lobed neutrophils or the presence of six-lobed neutrophils and macro-ovalocytes, were detected in 54 (55.7%) and 86 (88.7%) patients, respectively. Serum levels of vitamin B12 were < 50 pg/mL in 39 (40.2%) patients, 50 to 100 pg/mL in 33 (34.0%) patients, and 101 to 200 pg/mL in 25 (25.8%) patients. The median serum vitamin B12 level was 64.5 pg/mL (range, 10-198 pg/mL) (Table 3).
Serum antibody to intrinsic factor was detected in 62 (77.5%) of 80 patients examined. Serum antibody to parietal cells was detected in 35 (43.2%) of 81 patients examined. Both serum antibody to intrinsic factor and serum antibody to parietal cells were detected in 27 (34.6%) of 78 patients examined. Either one of the two serum antibodies was detected in 71 (85.5%) of 83 patients examined (Table 4). Of the 97 patients, 84 underwent endoscopic examination. Fifty-six of 57 (98.2%) patients who underwent gastric biopsy had atrophic gastritis of the fundus or body of the stomach. One patient had gastric adenoma. H. pylori was demonstrated in 7 (20.6%) of 34 patients examined by Giemsa staining of endoscopic biopsy specimens (Table 4).
Eighty-three (94.3%) of 88 patients who were treated by intramuscular injection of cobalamin normalized their hemoglobin levels within 3 months of treatment. The WBC counts returned to normal values in 40 (90.9%) of 44 patients, and platelet counts returned to normal values in 56 (88.9%) of 63 patients. After cobalamin therapy, the vast majority of symptoms, including generalized weakness, exertional dyspnea, vertigo, sore tongue, and anorexia, were improved in all patients; however, numbness was improved in only 17 (47.2%) of 36 patients (Table 5).
The present study describes the largest number of Korean individuals with pernicious anemia. Between 1995 and 2010, a total of 97 patients with pernicious anemia were enrolled from five university hospitals in one Korean province, which was nearly one-fourth of patients with megaloblastic anemia induced by vitamin B12 deficiency. In Western countries, prevalence and annual incidence of pernicious anemia have been reported to be about 150 per 100,000 and approximately 10 per 100,000, respectively (10, 11). During the period between 2005 and 2010 in the present study, the approximate annual incidence of pernicious anemia was 0.3 per 100,000, indicating that this disorder is much less frequent in Korea than in Western countries. Based on the current clinical practice situation in Chungnam province, we believe that the vast majority of patients with pernicious anemia were seen at the five hospitals that participated in the present study. However, one university hospital did not participate in the study, and some patients may have visited hospitals outside this area. Thus, the incidence of pernicious anemia is likely to be even higher than that estimated here.
In the present study, total gastrectomy was found to be a leading cause of vitamin B12 deficiency anemia, which differs from Western reports. One possible explanation for this discrepancy is that gastrectomy has been practiced more frequently in Korea than in Western countries because of a higher incidence of gastric cancer and H. pylori-associated gastric ulcers. Our study revealed that a proportion of patients who undergo total gastrectomy have not been placed on appropriate cobalamin replacement, despite the fact that monitoring and management for vitamin B12 deficiency is a well-known practice in individuals who undergo total gastrectomy.
The present study showed that most patients had symptoms of anemia and gastrointestinal manifestations, and about one-third of patients had neurologic symptoms at presentation. After cobalamin treatment the gastrointestinal symptoms resolved completely in all patients, whereas neurological symptoms remained in some. These features in Korean patients do not differ from those in Western cases (1). Pernicious anemia may be associated with autoimmune endocrinopathies and antireceptor autoimmune disease such as chronic autoimmune thyroiditis (Hashimoto's thyroiditis), insulin-dependent diabetes mellitus, Addison's disease, primary ovarian failure, primary hypoparathyroidism, Graves' disease, vitiligo, myasthenia gravis, and the Lambert-Eaton syndrome (1). In the present study, pernicious anemia was accompanied by several autoimmune disorders. However, the prevalence of autoimmune disorders in our study population was lower than that in Western populations. For example, autoimmune thyroid diseases were present in 10.2% of our study population, which is much lower than the 24% to 27% in Western populations (12, 13). At the present time, we have no explanation for this difference; thus, further studies are warranted.
Pernicious anemia is known to be associated with gastric adenocarcinoma as well as gastric carcinoid tumors. In a population-based cohort study in Sweden, the risk of gastric carcinoma was increased 3-fold and that of gastric carcinoid tumors was increased 13-fold in patients with pernicious anemia (14, 15). We found gastric adenocarcinoma in two patients at the time of diagnosis of pernicious anemia and in two more patients during follow-up. Because the present study was a retrospective analysis, some cases of gastric cancer were possibly not detected. Given the prevalence of gastric cancer in general population in Korea (16), it is suggested that pernicious anemia might be a risk factor for the development of gastric cancer in Korea as well.
No institutes that participated in this study used the Schilling test for the diagnosis of pernicious anemia. As an antibody assay is noninvasive, simple, and capable of differentiating cobalamin malabsorption due to intrinsic factor deficiency from other causes such as cobalamin malabsorption, measurement of serum antibody to intrinsic factor now supersedes the Schilling test in terms of confirmation of the diagnosis of pernicious anemia (1, 17). The frequencies of serum antibodies to intrinsic factor and parietal cells in this study were 77.5% and 43.2%, respectively. These profiles are similar to those among Western cases with pernicious anemia, in which the frequencies of serum antibodies to intrinsic factor and parietal cells are about 70% and 35% to 40%, respectively (1). In the past, anti-parietal cell antibody was detected more frequently than anti-intrinsic factor antibody (18, 19). The disparities could be explained, at least in part, by recent improvement in the detection of anti-intrinsic factor antibody. Furthermore, it has been hypothesized that the frequency of anti-parietal cell antibody is decreased by loss of antigen, which is caused by loss of parietal cells according to the progression of autoimmune gastritis (20).
There has been a notion that H. pylori infection provokes type A gastritis and thus induces pernicious anemia (21, 22). On the other hand, contradictory observations have also been reported (23, 24). In the present study, only 7 (20.6%) of 34 patients were positive in H. pylori testing, which is a rather low positivity compared with the 60% to 70% positivity in the general Korean population (25, 26). Thus, H. pylori infection is unlikely to be associated with pernicious anemia.
In conclusion, pernicious anemia is much less frequent in Koreans than in Western populations; however, the clinical features of this disorder in Koreans do not differ from those of Western cases.
Figures and Tables
Fig. 1
Annual distribution of the numbers of newly diagnosed patients with cobalamin deficiency anemia and pernicious anemia in Daejeon City and Chungnam Province.
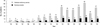
References
1. Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997. 337:1441–1448.
2. Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997. 84:223–243.
3. Carmel R. Ethnic and racial factors in cobalamin metabolism and its disorders. Semin Hematol. 1999. 36:88–100.
4. Sugihara T, Yawata Y. Japanese clinical statistical data of patients with pernicious anemia. Nihon Rinsho. 1992. 50:771–786.
5. Wun Chan JC, Yu Liu HS, Sang Kho BC, Yin Sim JP, Hang Lau TK, Luk YW, Chu RW, Fung Cheung FM, Tat Choi FP, Kwan Ma ES. Pernicious anemia in Chinese: a study of 181 patients in a Hong Kong hospital. Medicine (Baltimore). 2006. 85:129–138.
6. Yim PS, Woo MS, Chung SJ, Yoon YS, Jeen TH, Lee YB. A case report of pernicious anemia. Korean J Hematol. 1969. 4:23–31.
7. Lee DG, Do IH, Kim DW, Park JI, Chung SY, Moon SK. A case of pernicious anemia associated with chronic atrophic gastirtis. Korean J Hematol. 1987. 22:115–121.
8. Song HH, Kwon JH, Kim JH, Jeong JY, Kim HJ, Lee KS, Jang DY, Ahn JS, Shin DH, Kang SH, et al. Causes and clinical features of vitamine B12 deficiency megaloblastic anemia. Korean J Hematol. 2004. 39:243–248.
9. Chun JM, Park NS, Park NH, Yun GW, Yang YJ, Park SE, Yun HJ, Jo DY, Kwon GC, Kim S. Pernicious anemia: a retrospective analysis of 22 cases. Korean J Hematol. 2005. 40:219–225.
10. Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune disease in the United States. Clin Immunol Immunopathol. 1997. 84:223–243.
11. Pedersen AB, Mosbech J. Morbidity of pernicious anemia: incidence, prevalence, and treatment in a Danish country. Acta Med Scand. 1969. 185:449–452.
12. Carmel R, Spencer CA. Clinical and subclinical thyroid disorders associated with pernicious anemia. Observations on abnormal thyroid-stimulating hormone levels and on a possible association of blood group O with hyperthyroidism. Arch Intern Med. 1982. 142:1465–1469.
13. Ottesen M, Feldt-Rasmussen U, Andersen J, Hippe E, Schouboe A. Thyroid function and autoimmunity in pernicious anemia before and during cyanocobalamin treatment. J Endocrinol Invest. 1995. 18:91–97.
14. Hsing AW, Hansson LE, McLaughlin JK, Nyren O, Blot WJ, Ekbom A, Fraumeni JF Jr. Pernicious anemia and subsequent cancer. A population based cohort study. Cancer. 1993. 71:745–750.
15. Karlson BM, Ekbom A, Wacholder S, McLaughlin JK, Hsing AW. Cancer of the upper gastrointestinal tract among patients with pernicious anemia: a case-cohort study. Scand J Gastroenterol. 2000. 35:847–851.
16. Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2008. Cancer Res Treat. 2011. 43:1–11.
17. Carmel R. Pepsinogens and other serum markers in pernicioua anemia. Am J Clin Pathol. 1988. 90:442–445.
18. Irvine WJ. Immunologic aspects of pernicious anemia. N Engl J Med. 1965. 273:432–438.
19. Samloff IM, Kleinman MS, Turner MD, Sobel MV, Jeffries GH. Blocking and binding antibodies to intrinsic factor and parietal call antibody in pernicious anemia. Gastroenterology. 1968. 55:575–583.
20. Toh BH, Alderuccio F. Pernicious anaemia. Autoimmunity. 2004. 37:357–361.
21. Kaptan K, Beyan C, Ural AU, Cetin T, Avcu F, Gülşen M, Finci R, Yalçín A. Helicobacter pyroli is it a novel causative agent in vitamin B12 deficiency? Arch Intern Med. 2000. 160:1349–1353.
22. Stopeck A. Links between Helicobacter pylori infection, cobalamin deficiency, and pernicious anemia. Arch Intern Med. 2000. 160:1229–1230.
23. Haruma K, Komoto K, Kawaguchi H, Okamoto S, Yoshihara M, Sumii K, Kajiyama G. Pernicious anemia and Helicobacter pyroli infection in Japan. evaluation in a country with a high prevalence of infection. Am J Gastroenterol. 1995. 90:1107–1110.
24. Fong TL, Dooley CP, Dehesa M, Cohen H, Carmel R, Fitzgibbons PL, Perez-Perez GI, Blaser MJ. Helicobacter pyroli infection in pernicious anemia. a prospective controlled study. Gastroenterology. 1991. 100:328–332.
25. Kim HS, Lee YC, Lee HW, Yoo HM, Lee CG, Kim JM, Lee KJ, Kim PS, Moon BS, Park HJ, et al. Seroepidemiologic study of Helicobacter pyroli infection in Korea. Korean J Gastroenterol. 1999. 33:170–182.
26. Yim JY, Kim N, Choi SH, Kim YS, Cho KR, Kim SS, Seo GS, Kim HU, Baik GH, Sin CS, et al. Seroprevalence of Helicobacter pyroli in South Korea. Helicobacter. 2007. 12:333–340.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download