Abstract
We evaluated long-term results of De Vega annuloplasty measured by cylindrical sizers for functional tricuspid regurgitation (FTR) and analyzed the impact of measured annular size on the late recurrence of tricuspid valve regurgitation. Between 2001 and 2011, 177 patients (57.9±10.5 yr) underwent De Vega annuloplasty for FTR. Three cylindrical sizers (actual diameters of 29.5, 31.5, and 33.5 mm) were used to reproducibly reduce the tricuspid annulus. Long-term outcomes were evaluated and risk factor analyses for the recurrence of FTR ≥3+ were performed. Measured annular diameter indexed by patient's body surface area was included in the analyses as a possible risk factor. Operative mortality occurred in 8 patients (4.5%). Ten-year overall and cardiac death-free survivals were 80.5% and 90.8%, respectively. Five and 10-yr freedom rates from recurrent FTR were 96.5% and 93.1%, respectively. Cox proportional hazard model revealed that higher indexed annular size was the only risk factor for the recurrence of FTR (P=0.006). A minimal P value approach demonstrated that indexed annular diameter of 22.5 mm/m2 was a cut-off value predicting the recurrence of FTR. De Vega annuloplasty for FTR results in low rates of recurrent FTR in the long-term. Tricuspid annulus should be reduced appropriately considering patients' body size to prevent recurrent FTR.
Previous studies demonstrated that tricuspid annuloplasty using annular sutures or insertion of a prosthetic ring resulted in favorable results for patients with functional tricuspid regurgitation (TR) (1-6). Among those, De Vega procedure is a well-known technique of suture annuloplasty correcting TR (1). However, there has been a concern that long-term results of De Vega annuloplasty are less satisfactory than those of annuloplasty with prosthetic rings (7, 8). In our institution, cylindrical sizers have been used during the De Vega annuloplasty to reproducibly measure the reduced tricuspid annular diameter, as suggested by another author (9). The aims of this study were 1) to evaluate the long-term results of measured De Vega annuloplasty and 2) to analyze the impact of the measured diameter of the tricuspid annulus on the late recurrence of TR.
From January 2001 to December 2011, 177 patients (male:female=63:114) who underwent De Vega procedure with or without concomitant cardiac surgery by a single surgeon were enrolled in the present study. Patients who had a history of previous tricuspid valvuloplasty and those with organic tricuspid valve pathology were excluded. Mean age at the operation was 57.9±10.5 yr. Hypertension was the most common co-morbidity (Table 1).
All operations were performed under a routine aorto-bicaval cannulation, moderate hypothermia and cold blood cardioplegic arrest via a median sternotomy. Mean cardiopulmonary bypass and aortic cross clamp times were 196±60 and 135±44 min, respectively. One hundred and sixty seven patients (94.4%) underwent concomitant cardiac procedures including mitral valve operation (n=155), arrhythmia surgery (n=84), and aortic valve procedure (n=53) (Table 2).
De Vega annuloplasty was performed with a 3-0 extended polytetrafluoroethylene (e-PTFE) suture. Reduced annular size was measured with commercially available cylindrical valve sizers. Three sizers with actual diameters of 29.5, 31.5, and 33.5 mm (labeled sizes of 27, 29, and 31 mm) were used. Annular reduction was performed by tying down the plication suture while the cylindrical sizer was inserted in the tricuspid valve orifice. In early study period, a sizer with an actual diameter of 33.5 mm was used due to a concern of too tight reduction (n=37). However, since 2003, a sizer with diameter of 31.5 mm was used in the majority of the patients (n=129) by the operating surgeon's choice. During the study period, De Vega technique was exclusively used for all patients of the operating surgeon who needed tricuspid annuloplasty.
Patients underwent regular postoperative follow-up through the outpatient clinic at 3 or 4 month intervals, and were contacted by telephone for confirmation of their condition if the last clinic visit was not conducted at the scheduled time. Clinical follow-up was closed on December 31, 2012. Follow-up was complete in 96.6% (171 of 177) of patients, with a follow-up duration of 72±36 months. Operative mortality was defined as death within 30 days or during the same hospitalization after the surgery. Cardiac death was defined as any death related to cardiac events, including sudden death during the follow-up. Valve-related complications were recorded according to the previous guidelines (10). Tricuspid valve-related events (TVRE) included the following: 1) Cardiac death, 2) recurrent TR ≥3+, 3) tricuspid valve endocarditis, 4) tricuspid valve reoperation, and 5) congestive heart failure needed readmission.
An initial postoperative echocardiographic evaluation (7 [1-46] days after surgery) was performed before discharge in all but 3 patients who died early after the surgery. Follow-up echocardiograms were performed at the discretion of the operating surgeon or referring physicians during the follow-up. At least 1 follow-up echocardiogram after the early postoperative period was performed in 94.1% (159 of 169) of the survivors. The last follow-up echocardiogram was performed 57±34 months after the surgery. Tricuspid regurgitation was graded from 0 to 4+; 0=no, 1+=mild, 2+=moderate, 3+=moderately severe, and 4+=severe. Recurrent TR was defined as TR of equal or more than grade 3 after the surgery.
Statistical analysis was performed using the SAS statistical software (SAS system for Windows, version 9.2; SAS institute, Cary, NC, USA) and the SPSS software package (Version 12.0, SPSS Inc, Chicago, IL, USA). Data were expressed as mean±standard deviation, median with ranges, or proportions. Comparisons of categorical and continuous variables were performed with the chi-squared test or Fisher exact test and Student t test, respectively. Multivariate analysis to identify risk factors for operative mortality was performed with logistic regression analysis. Survival rates were estimated using the Kaplan-Meier method. The Cox proportional hazard model was adopted for analysis of risk factors for recurrent TR. The proportional hazard property was tested using the restricted cubic spline (11, 12). All independent variables in the Cox regressions were met the proportional hazards assumption. When the separation occurred in univariate analysis, hazard ratio (HR) was estimated using penalized maximum likelihood test. It was demonstrated with 95% confidence interval (CI). Multicollinearity was controlled using backward stepwise regression. Variables with a P value of less than 0.2 were entered into multivariate analyses. To analyze the impact of selection of the diameter of the cylindrical sizers, the diameter of the sizers (=reduced annular diameter) was included in the analysis. In addition, tricuspid valve orifice index (TVOI) was defined as a reduced annular diameter divided by the patient's body surface area and included in the analysis. The minimal P value approach was used to estimate an optimal cut-off value of a continuous variable predicting a time-related event (13). A P value of less than 0.05 was considered statistically significant.
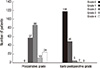
The operative mortality rate was 4.5% (8 of 177). Postoperative morbidities included low cardiac output syndrome (n=24, 13.6%), postoperative bleeding requiring reoperation (n=11, 6.2%), stroke (n=9, 5.1%), and acute renal failure (n=5, 2.8%) (Table 3). Multivariate analysis revealed that combined coronary artery disease was the only significant risk factor for early mortality (Table 4). Early postoperative echocardiography revealed TR grades of 2+ and 3+ in 5 and 2 patients, respectively (Fig. 1).
Among 169 survivors, late death occurred in 19 (11.2%) patients including 8 cardiac deaths. Overall survival rates at 5 and 10 yr were 85.5% and 80.5%, respectively. Age (HR [95% CI]=1.062 [1.023-1.103], P=0.001), smoking (HR [95% CI]=3.835 [1.522-9.664], P=0.004) and coronary artery disease (HR [95% CI]=5.232 [1.136-24.087], P=0.034) were associated with overall survival.
Freedom rates from cardiac death at 5 and 10 yr were 94.3% and 90.8%, respectively. Chronic renal failure (HR [95% CI]=13.255 [2.204-79.740], P=0.005), coronary artery disease (HR [95% CI]=14.951 [2.265-98.689], P=0.005) and smoking (HR [95% CI]=6.189 [1.499-25.555], P=0.012) were risk factors for long-term cardiac death.
Tricuspid valve regurgitation was found in 7 patients during the follow-up period. Freedom rates from TR ≥3+ at 5 and 10 yr were 96.5% and 93.1%, respectively (Fig. 2). None of the study patients had significant tricuspid valve stenosis during the follow-up. Mean transvalvular pressure gradient of repaired tricuspid valve was 1.06±0.45 mmHg.
Tricuspid valve reoperation was performed in 2 patients. One patient underwent mitral and tricuspid valve replacement at 1 month after the initial mitral repair and tricuspid annuloplasty due to persistent severe mitral regurgitation and moderately severe TR. The other patient underwent tricuspid valve replacement 29 months after the surgery due to severe TR. Reoperative findings in both patients revealed intact initial De Vega sutures. The patients' TVOIs were 22.64 and 23.16 mm/m2, respectively. Readmission owing to congestive heart failure was needed in 9 patients. Although 3 patients experienced infective endocarditis, those were not related with the tricuspid valve. No patient suffered from thromboembolism from the right heart chamber. Five- and 10-yr TVRE-free survivals were 91.3% and 81.9%, respectively. Age (HR [95% CI]=1.090 [1.037-1.146], P=0.001) and coronary artery disease (HR [95% C I]=9.977 [1.781-55.870], P=0.009) but not TVOI were associated with TVRE-free survival.
During the follow-up, left-sided valve-related events occurred in 10 patients including severe regurgitation of repaired mitral valve (n=1), structural valve deterioration of a bioprosthetic mitral valve (n=1), paravalvular leak of prosthetic mitral valves (n=4), and infective endocarditis (n=4). Among those, 7 patients underwent mitral and aortic valve reoperations. At last follow-up, atrial fibrillation remained in 74 patients including 22 of 84 patients who underwent concomitant arrhythmia surgery. Left ventricular dimensions and systolic pulmonary artery pressure improved significantly at the last follow-up echocardiography. However, there was no change in left ventricular ejection fraction (Table 5).
By univariate risk factor analyses, old age, female sex, small body mass index, chronic renal failure, preoperative TR grade and the TVOI were entered into a multivariate analysis. Body surface area was excluded to control the multi-collinearity between TVOI and body surface area. Difference in diameter of the cylindrical sizers was not associated with the recurrence of TR (P=0.489). Recurrence of the left-sided valve problem was also not a risk factor for the recurrence of TR (P=0.302). There was no late recurrence of significant TR in patients with TR < 2+, and preoperative TR grade was a significant factor associated with late recurrence of TR in the univariate analysis. However, this significance disappeared in the multivariate analysis. In backward stepwise multivariate analysis, only TVOI was left in the final model as a significant factor (P=0.006, Hazard ratio [95% confidence interval]=1.80 [1.18-2.75]) (Table 6). A cut-off value of TVOI to predict the recurrence of TR was analyzed by the minimal P value approach. A TVOI of 22.5 mm/m2 was the best cut-off value to predict the recurrence of TR (Fig. 3). In other words, the tricuspid valve annulus should be reduced according to the equation as follows; reduced tricuspid valve orifice diameter<22.5×patient's body surface area.
This study demonstrated 2 main findings. First, long-term results of the De Vega annuloplasty for functional TR were favorable with 10-yr freedom rates from recurrent TR and TVRE of 93.1% and 81.9%, respectively. Second, annular reduction should be performed considering patients' body size to optimize long-term results of the De Vega annuloplasty.
Since 1965, various surgical techniques of tricuspid annuloplasty have been introduced for functional TR. These included suture plication, and annuloplasty using a pericardial strip, flexible band and rigid rings (1-3, 14). Although the De Vega annuloplasty has been widely performed for functional TR in various conditions, there has been a concern that long-term results of the De Vega procedure is less satisfactory than those of the prosthetic ring annuloplasty (6-8). Supporters of a ring annuloplasty have insisted that the use of a ring provides several advantages including equal distribution of tension, standardized annular reduction, ease of learning and reproducibility (15). Two studies, conducted in the same center, consistently reported that tricuspid valve repair without a prosthetic ring was associated with late recurrence of TR (7, 8). On the contrary, Morishita and his colleagues reported that the De Vega annuloplasty is a safe and effective method to treat functional TR with a 10-yr reoperation-free rate of 94.4% (16). In the present study, the 10-yr freedom rate from TR ≥3+ were 93.1%, and only 2 patients underwent redo-tricuspid valve operation during the follow-up period. When performing the annular reduction, we used cylindrical sizers to reproducibly reduce the tricuspid annulus. In addition, the e-PTFE suture was used with an expectation, although evidence is lacking, that it could prevent suture breakdown and dehiscence (so called bow-string effect), which has been found when using the polypropylene suture (17).
Previous studies demonstrated that preoperative TR grade, severe left ventricular dysfunction, and preoperative TV tethering caused by dilated right ventricle were identified as predictors of recurrent TR (3, 18, 19). In the present study, however, preoperative TR grade and left ventricular dysfunction were not associated with the recurrence of TR. To evaluate whether reduced annular diameter could affect results of annuloplasty, we included measured diameter by the cylindrical sizer and indexed measured diameter (TVOI) as possible risk factors. The univariate and multivariate analyses revealed that not the reduced diameter of the tricuspid annulus itself but indexed annular diameter by body surface area was associated with the recurrence of TR. The minimal P value approach, which was a statistical method to identify a cut-off value in time-related events, revealed that an indexed annular diameter of 22.5 mm/m2 was a cut-off value to warrant annuloplasty failure.
Interestingly, we could evaluate the fate of initial De Vega sutures in two patients who underwent tricuspid reoperation during the follow-up period. Tricuspid regurgitation recurred even though previous e-PTFE sutures were intact. The TVOIs in these patients were 22.64 and 23.16 mm/m2, respectively. This might reflect that the optimal sizing of annular reduction according to the patient's size is necessary in order to prevent the late recurrence of TR. Further longer-term follow up data to evaluate the clinical benefit of restrictive De Vega procedure and prospective randomized study comparing between tight ring annuloplasty and restrictive De Vega procedure are needed in the near future.
There are several limitations to the present study that must be recognized. First, the present study was a retrospective observational study in a single institution. Second, echocardiography was not performed on a regular interval during the follow-up. Third, we did not evaluate preoperative tricuspid annular diameter and leaflet tethering, due to retrospective nature of this study. Fourth, precise indications of tricuspid annuloplasty were not described due to the retrospective nature of this study.
Figures and Tables
Fig. 3
A Kaplan-Meier curve for freedom from tricuspid regurgitation (TR) ≥3+ according to the cut-off value of tricuspid valve orifice index (TVOI).

ACKNOWLEDGEMENTS
We wish to thank the Medical Research Collaborating Center of the Seoul National University Hospital for their efforts in statistical assistance.
References
1. Holper K, Haehnel JC, Augustin N, Sebening F. Surgery for tricuspid insufficiency: long-term follow-up after De Vega annuloplasty. Thorac Cardiovasc Surg. 1993; 41:1–8.
2. Kay JH, Maselli-Campagna G, Tsuji KK. Surgical treatment of tricuspid insufficiency. Ann Surg. 1965; 162:53–58.
3. McCarthy PM, Bhudia SK, Rajeswaran J, Hoercher KJ, Lytle BW, Cosgrove DM, Blackstone EH. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg. 2004; 127:674–685.
4. Burma O, Ustunsoy H, Davutoglu V, Celkan MA, Kazaz H, Pektok E. Initial clinical experience with a novel biodegradable ring in patients with functional tricuspid insufficiency: Kalangos Biodegradable Tricuspid Ring. Thorac Cardiovasc Surg. 2007; 55:284–287.
5. Navia JL, Nowicki ER, Blackstone EH, Brozzi NA, Nento DE, Atik FA, Rajeswaran J, Gillinov AM, Svensson LG, Lytle BW. Surgical management of secondary tricuspid valve regurgitation: annulus, commissure, or leaflet procedure? J Thorac Cardiovasc Surg. 2010; 139:1473–1482.e5.
6. Guenther T, Mazzitelli D, Noebauer C, Hettich I, Tassani-Prell P, Voss B, Lange R. Tricuspid valve repair: is ring annuloplasty superior? Eur J Cardiothorac Surg. 2013; 43:58–65.
7. Tang GH, David TE, Singh SK, Maganti MD, Armstrong S, Borger MA. Tricuspid valve repair with an annuloplasty ring results in improved long-term outcomes. Circulation. 2006; 114:I577–I581.
8. Sales VL, McCarthy PM. Durability of functional tricuspid valve repair. Semin Thorac Cardiovasc Surg. 2010; 22:97–103.
9. Antunes MJ. De Vega annuloplasty of the tricuspid valve. Oper Tech Thorac Cardiovasc Surg. 2003; 8:169–176.
10. Akins CW, Miller DC, Turina MI, Kouchoukos NT, Blackstone EH, Grunkemeier GL, Takkenberg JJ, David TE, Butchart EG, Adams DH, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. J Thorac Cardiovasc Surg. 2008; 135:732–738.
11. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989; 8:551–561.
12. Hess KR. Assessing time-by-covariate interactions in proportional hazards regression models using cubic spline functions. Stat Med. 1994; 13:1045–1062.
13. Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using "optimal" cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. 1994; 86:829–835.
14. Chang BC, Song SW, Lee S, Yoo KJ, Kang MS, Chung N. Eight-year outcomes of tricuspid annuloplasty using autologous pericardial strip for functional tricuspid regurgitation. Ann Thorac Surg. 2008; 86:1485–1492.
15. Chikwe J, Anyanwu AC. Surgical strategies for functional tricuspid regurgitation. Semin Thorac Cardiovasc Surg. 2010; 22:90–96.
16. Morishita A, Kitamura M, Noji S, Aomi S, Endo M, Koyanagi H. Long-term results after De Vega's tricuspid annuloplasty. J Cardiovasc Surg (Torino). 2002; 43:773–777.
17. Revuelta JM, Garcia-Rinaldi R. Segmental tricuspid annuloplasty: a new technique. J Thorac Cardiovasc Surg. 1989; 97:799–801.
18. Fukuda S, Song JM, Gillinov AM, McCarthy PM, Daimon M, Kongsaerepong V, Thomas JD, Shiota T. Tricuspid valve tethering predicts residual tricuspid regurgitation after tricuspid annuloplasty. Circulation. 2005; 111:975–979.
19. Fukuda S, Gillinov AM, McCarthy PM, Matsumura Y, Thomas JD, Shiota T. Echocardiographic follow-up of tricuspid annuloplasty with a new three-dimensional ring in patients with functional tricuspid regurgitation. J Am Soc Echocardiogr. 2007; 20:1236–1242.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download