This article has been corrected. See "Erratum: Correction of the Grant Number" in Volume 29 on page 616.
Abstract
Non-human primate studies must be conducted prior to the clinical trial of xenotransplantation. In order to develop clinically applicable immune-modulatory regimen through non-human primate studies, close monitoring of xenogeneic immune responses is required. We adopted multiplex cytokine analysis in assessment of the immune status during the course of pig-to-non-human primate islet transplantation. This study aimed to assess the feasibility of this multiplex cytokine assay in the development of immune-modulatory regimen. Using this assay, we were able to detect different cytokines with a minimal usage of blood samples, and this allowed us to detect various immunological situations in the recipients. Detection of TNF-α surge (347.8 pg/mL) guided us to block TNF-α in the early phase of transplantation. Supportive information for in vivo efficacy of cytokine neutralizing antibody could be speculated by in vitro neutralization assay (1,250 pg/mL → 0 pg/mL). In addition, periodic monitoring of cytokines in peripheral blood allowed the detection of the infection episode prior to other routine assays. These benefits of multiplex cytokine assay may be generally applied to other pre-clinical research, which is a prerequisite for clinical trials.
To accomplish 'bench-to-bedside' translational medicine, novel therapeutic tools developed in basic research must be confirmed in non-human primates before their application to humans. Xenotransplantation, which is utilized to overcome the shortage of organ donors is not an exception in terms of this principle. 'First WHO Global Consultation on Regulatory Requirements for Xenotransplantation Clinical Trials, The Changsha Communiqué' declared that non-human primate testing for safety and efficacy is a prerequisite for clinical trials using xenotransplantation products (1). In the case of xenogeneic pancreatic islet transplantation, a successful non-human primate study that results in a stable maintenance of normoglycemia beyond 6 months is required (2). However, previous non-human primate studies showed that either porcine islets were promptly rejected, or potent immunosuppressants were required for their maintenance (3). Since detrimental regimen cannot be approved for clinical trials, clinically applicable immunosuppressive drug regimen must be developed (4). To develop appropriate combination of immune-modulatory agents, the effects of each agent should be evaluated by closely monitoring the immune status.
In this study, it was aimed to assessing the feasibility of multiplex cytokine analysis in the development of immune-modulatory regimen. Blood cytokine levels were monitored via multiplex cytokine assay during the course of pig-to-non-human primate islet transplantation. Changes in the level of various cytokines must be considered for different conditions of xenogeneic immune responses. By applying multiplex analysis, which requires just a single experiment, we were able to circumvent the requirement for large amount of samples to analyze the diverse cytokines. In addition, the immunological episodes in recipient monkeys were predicted by periodic evaluation of blood cytokine levels by incorporating this assay.
Rhesus macaque monkeys imported from China were used in this study. Seoul National University (SNU) miniature pigs were bred and maintained in a specific pathogen-free facility. All animal studies were performed after receiving approval of the Institutional Animal Care and Use Committee (IACUC) in Seoul National University Hospital (IACUC approval No. 13-2010-000-6).
Total pancreas of SNU miniature pigs was obtained in a sterile operating room. Islet isolation was performed using the modified Ricordi method as previously described (5). Laparotomy was performed on diabetic recipient monkey under general anesthesia, and porcine islets were infused through a catheter which was inserted into the jejunal vein and approached near the portal vein.
Peripheral blood obtained from monkeys was centrifuged to acquire plasma for cytokine analysis. Cytometric Bead Array (CBA) Non-human Primate Th1/Th2 Cytokine Kit (BD Biosciences, San Jose, CA, USA) was used to detect IL-2, IL-4, IL-5, IL-6, TNF-α, and IFN-γ in a single sample. As the manufacturer's instruction, antibody-coated beads for each cytokines were mixed and incubated with plasma samples and PE-detection antibodies for 3 hr at room temperature. After washing, samples were acquired with a FACSCanto II (BD Biosciences) and analyzed with FACSDiva software (BD Biosciences). Cytokine concentration of each sample was calculated with the standard curve acquired with fixed concentrations of standard cytokine samples.
For in vitro neutralization test of anti-TNF-α antibody drug, Humira (AbbVie Inc. North Chicago, IL, USA), multiplex cytokine assay was conducted after 1 hr pre-incubation of standard cytokine sample with 20 µg/mL of Humira.
We attempted to set up an effective analytical method to develop clinically applicable immune-modulatory regimen to control xenogeneic immune responses to the porcine islets transplanted through the portal vein route in diabetic Rhesus macaque monkey. The multiplex cytokine assay provided supportive information for seeking the best combination of immune-modulatory agents. In a diabetic recipient monkey (R048), the blood glucose level was normalized shortly after the porcine islets transplantation, but became unstable at day 5 and eventually returned to a hyperglycemic state similar to pre-transplantation level on day 12 (Fig. 1A). Multiplex cytokine assay using periodic blood samples from this monkey clearly detected tumor necrosis factor (TNF)-α surge through day 7 to 14 (Fig. 1B). When the porcine islets were completely rejected as revealed by the absence of porcine C-peptide and persistent hyperglycemia, TNF-α cytokine level was normalized to the basal level in the plasma of peripheral blood. Since this result implied a critical role of TNF-α in the acute rejection process, we decided to incorporate anti-TNF-α neutralizing antibody drug, Humira (Adalimumab) during the peri-transplant period in subsequent experiments.
Before using Humira in the porcine islet transplantation experiment, we explored the possibility of predicting in vivo neutralizing efficacy of Humira with multiplex cytokine assay. The cytokine standard provided by the manufacturer contained 6 different cytokines including TNF-α. Pre-incubation of cytokines with 20 µg/mL of Humira for 1 hr specifically blunted TNF-α signal in FACS diagram, and even the highest concentration of TNF-α (1,250 pg/mL) was completely neutralized (Fig. 2). Therefore, we predicted that in vivo neutralizing effect of TNF-α by Humira and further incorporation of Humira into the immune suppressive regimen on the day of transplantation would abrogate TNF-α-mediated detrimental effects towards the porcine islets.
One recipient monkey (R042) which had rejected the porcine islets was selected as a subject for re-transplantation experiment. Before re-transplanting with porcine islets, basal immune status was assessed using multiplex cytokine assay. Interestingly, high concentrations of both IL-2 and IFN-γ were detected on the day before islet re-transplantation (Fig. 3A). Since IL-2 and IFN-γ are the typical cytokines of type 1 T helper (Th1) cells, it is likely that Th1 response associated with previous islet transplantation was not completely resolved. In line with this speculation, Th1 cells are considered as key players in graft rejection (6). Although we had used the same immunosuppressive regimen that had been successful in the naïve recipient, this recipient rejected porcine islets within 5 days after islet re-transplantation (Fig. 3B). Collectively, the multiplex cytokine assay can be used to predict potential detrimental outcome after transplantation by measuring indicative cytokines in basal state prior to transplantation.
In one recipient monkey (R055), severe diarrhea and edema occurred on day 45 after porcine islet transplantation. Analysis of peripheral blood cytokines with multiplex cytokine assay from samples taken periodically during the follow-up period after transplantation showed sustained low levels of cytokines up to 34 days; however, an abrupt IL-6 surge appeared on day 46 (Fig. 4). Importantly, at that moment, systemic infection through the central venous catheter line was suspected. This IL-6 surge preceded renal and hepatic dysfunction which was confirmed by blood chemistry test and a significant increase of white blood cell count in complete blood count test (CBC) appeared on day 47 and day 51, respectively. The recipient eventually died on day 54 by septic shock, and abscess around the catheter was detected in autopsy. These results indicated that periodic monitoring of plasma cytokine by multiplex cytokine assay could predict the infection episode, exampled by infection through the central venous catheter line in case of the R055 monkey.
In this study, the usefulness of multiplex cytokine analysis was revealed in various immunological episodes during the course of pig-to-non-human primate islet transplantation. Owing to the wide coverage of the multiplex cytokine assay, TNF-α surge during acute rejection phase, insufficient clearance of Th1 cytokines after previous transplantation and IL-6 surge in an infection episode could be detected efficiently.
In Fig. 1, TNF-α surge could be detected during acute rejection phase of xenogeneic porcine islet transplantation. TNF-α is a pro-inflammatory cytokine which regulates immunogenicity of transplanted tissue by amplification of major histocomaptibility complex gene expression (7), and provokes rejection by a direct cytotoxic effect or via T cell or macrophage action (8-10). Indeed, the expression of TNF-α which peaked on day 5, was observed in heart allograft (11). Furthermore, evaluation of plasma level of TNF-α could be used as allograft rejection marker (12). This study suggests predictive value of blood TNF-α surge for detection of acute rejection in xenotransplantation setting for the first time.
In Fig. 2, potent neutralizing efficacy of anti TNF-α antibody drug, Humira could be observed. Humira specifically blocked the binding of TNF-α to capturing beads not affecting other cytokines. According to this result, we could predict its efficient in vivo function to block TNF-α during the acute phase of pig-to-non-human primate islet transplantation.
Although various cytokines must be considered for different immunological changes, multiplex analysis can circumvent the requirement for large amount of samples to analyze the diverse cytokines. Since periodic monitoring may require frequent sampling of blood, the multiplex cytokine assay which requires a minimum volume of blood has a great advantage in pre-clinical and clinical settings.
Recently, the anterior chamber of the eye was suggested as a novel transplantation site for pancreatic islets (13, 14). This route of transplantation has benefits in terms of direct visualization of the engraftment and rejection processes of transplanted islets (15). Indeed, a pre-clinical allogeneic islet transplantation was conducted in a baboon monkey (16). This route must also be monitored for immune responses in the sequestered local environment of the anterior chamber. However, the volume of aqueous humor in the anterior chamber is only 200 µL in human (17), which may be less in smaller non-human primates. Therefore, efficient multiplex cytokine analysis requiring a minimum volume of aqueous humor has advantage in the assessment of the immune response.
This study revealed the usefulness of multiplex cytokine analysis during the course of xenogeneic islet transplantation in non-human primates in detecting various immunological changes. This benefit may be generally applied to pre-clinical research which must be conducted ahead of clinical trials. However, small case number and specific setting of xenogeneic islet transplantation may prohibit the generalization. Therefore, feasibility of this assay must be confirmed in other settings with increased case numbers. Additionally, this blood cytokine monitoring may provide supportive information in care of future human recipients for porcine islet xenotransplantation.
Figures and Tables
Fig. 1
Detection of TNF-α surge in an episode of acute graft rejection. A recipient monkey of porcine islets was monitored for their diabetic status with blood glucose level, porcine c-peptide and exogenously injected insulin (A) and blood cytokine levels (B).
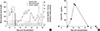
Fig. 2
Ex vivo test for neutralizing efficacy of antibody drug, Humira. Multiplex cytokine assay was conducted with (left panel) or without (right panel) pre-incubation with 20 µg/mL of anti-TNF-α neutralizing antibody, Humira. Beads for each cytokines are discerned with discrete APC-fluorescence intensities. PE-intensities of beads captured with detecting antibodies are depicted.

Notes
References
1. First WHO global consultation on regulatory requirements for xenotransplantation clinical trials: Changsha, China, 19-21 November 2008: the Changsha Communiqué. Xenotransplantation. 2009; 16:61–63.
2. Cooper DK, Casu A. The International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes: chapter 4: pre-clinical efficacy and complication data required to justify a clinical trial. Xenotransplantation. 2009; 16:229–238.
3. Marigliano M, Bertera S, Grupillo M, Trucco M, Bottino R. Pig-to-nonhuman primates pancreatic islet xenotransplantation: an overview. Curr Diab Rep. 2011; 11:402–412.
4. Van der Windt DJ, Bottino R, Kumar G, Wijkstrom M, Hara H, Ezzelarab M, Ekser B, Phelps C, Murase N, Casu A, et al. Clinical islet xenotransplantation: how close are we? Diabetes. 2012; 61:3046–3055.
5. Jin SM, Kim KS, Lee SY, Gong CH, Park SK, Yu JE, Yeom SC, Yoon TW, Ha J, Park CG, et al. Enhanced prediction of porcine islet yield and posttransplant outcome using a combination of quantitative histomorphometric parameters and flow cytometry. Cell Transplant. 2010; 19:299–311.
6. Atalar K, Afzali B, Lord G, Lombardi G. Relative roles of Th1 and Th17 effector cells in allograft rejection. Curr Opin Organ Transplant. 2009; 14:23–29.
7. Pujol-Borrell R, Todd I, Doshi M, Bottazzo GF, Sutton R, Gray D, Adolf GR, Feldmann M. HLA class II induction in human islet cells by interferon-gamma plus tumour necrosis factor or lymphotoxin. Nature. 1987; 326:304–306.
8. Sato N, Goto T, Haranaka K, Satomi N, Nariuchi H, Mano-Hirano Y, Sawasaki Y. Actions of tumor necrosis factor on cultured vascular endothelial cells: morphologic modulation, growth inhibition, and cytotoxicity. J Natl Cancer Inst. 1986; 76:1113–1121.
9. Ranges GE, Figari IS, Espevik T, Palladino MA Jr. Inhibition of cytotoxic T cell development by transforming growth factor beta and reversal by recombinant tumor necrosis factor alpha. J Exp Med. 1987; 166:991–998.
10. Philip R, Epstein LB. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986; 323:86–89.
11. Lee JR, Seok CJ, Kim JS, Chang JM, Seo JW. Expression of NF-kappaB and cytokines in chronic rejection of transplanted murine heart. J Korean Med Sci. 2001; 16:397–406.
12. Abdallah AN, Billes MA, Attia Y, Doutremepuich C, Cassaigne A, Iron A. Evaluation of plasma levels of tumour necrosis factor alpha and interleukin-6 as rejection markers in a cohort of 142 heart-grafted patients followed by endomyocardial biopsy. Eur Heart J. 1997; 18:1024–1029.
13. Speier S, Nyqvist D, Cabrera O, Yu J, Molano RD, Pileggi A, Moede T, Köhler M, Wilbertz J, Leibiger B, et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med. 2008; 14:574–578.
14. Speier S, Nyqvist D, Köhler M, Caicedo A, Leibiger IB, Berggren PO. Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nat Protoc. 2008; 3:1278–1286.
15. Abdulreda MH, Faleo G, Molano RD, Lopez-Cabezas M, Molina J, Tan Y, Echeverria OA, Zahr-Akrawi E, Rodriguez-Diaz R, Edlund PK, et al. High-resolution, noninvasive longitudinal live imaging of immune responses. Proc Natl Acad Sci U S A. 2011; 108:12863–12868.
16. Perez VL, Caicedo A, Berman DM, Arrieta E, Abdulreda MH, Rodriguez-Diaz R, Pileggi A, Hernandez E, Dubovy SR, Parel JM, et al. The anterior chamber of the eye as a clinical transplantation site for the treatment of diabetes: a study in a baboon model of diabetes. Diabetologia. 2011; 54:1121–1126.
17. Behndig A, Markström K. Determination of the aqueous humor volume by three-dimensional mapping of the anterior chamber. Ophthalmic Res. 2005; 37:13–16.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



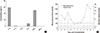

 XML Download
XML Download