This article has been corrected. See "Erratum: Correction of the Grant Number" in Volume 29 on page 615.
Abstract
Constructing a bone marrow chimera prior to graft transplantation can induce donor-specific immune tolerance. Mixed chimerism containing hematopoietic cells of both recipient- and donor-origin has advantages attributed from low dose of total body irradiation. In this study, we explored the mechanism of mixed chimerism supplemented with depletion of Natural Killer cells. Mixed chimerism with C57BL/6 bone marrow cells was induced in recipient BALB/c mice which were given 450 cGy of γ-ray irradiation (n = 16). As revealed by reduced proliferation and cytokine production in mixed leukocyte reaction and ELISpot assay (24.6 vs 265.5), the allo-immune response to bone marrow donor was reduced. Furthermore, the induction of transferable immunological tolerance was confirmed by adoptive transfer and subsequent acceptance of C57BL/6 skin graft (n = 4). CD4+FoxP3+ regulatory T cells were increased in the recipient compartment of the mixed chimera (19.2% → 33.8%). This suggests that regulatory T cells may be therapeutically used for the induction of graft-specific tolerance by mixed chimerism.
In order to conduct a successful allogeneic transplantation, it is crucial to control or minimize host immune responses to the allogeneic graft which leads to the failure of transplantation and therefore, rejection of the graft (1). The key players in graft rejection are immune cells which originate from bone marrow (BM), the primary lymphoid organ which possesses hematopoietic stem cells of immune cells (2, 3).
By replacing BM of recipient with that of donors, BM chimerism is able to induce successful graft survival in experimental models (4). It has subsequently been shown that constructing a BM chimera prior to graft transplantation is the most reliable method to induce immune tolerance in humans (5). This way, it becomes possible to induce the donor-specific tolerance for donor-derived graft (6, 7).
There are two types of chimerism. One is 'full chimerism' in which virtually all hematopoietic cells are of donor origin, and the other is 'mixed chimerism' in which hematopoietic cells of both recipient- and donor-origin co-exist (8). Although total body irradiation (TBI) is required in prior to BM transplantation, the high dose of TBI for the induction of full chimerism is clinically inapplicable (9). There are several advantages of mixed chimersim over full chimerism in terms of non-myeloablation, immunocompetence and intra-thymic deletion of both host- and donor-reactive T cells (10).
However, in many cases where recipients are given a low dose of TBI for the induction of mixed chimerism, the engraftment of transplanted BM frequently results in a failure. It has been demonstrated this failure of the engraftment is due to the presence of recipient-derived natural killer (NK) cells left after low dose TBI (11). For this reason, depletion of NK cells prior to TBI has been devised to improve the survival and engraftment of BM (12).
In parallel, we conducted experiments by using BM mixed chimeric mouse models to determine improved outcomes of immunological tolerance following the allogeneic transplantation. For NK-depletion, we used the anti-asialo GM1 antibody which is well known to selectively eliminate NK cells (13). In this study, we induced mixed chimerism with a reduced dose of TBI and transferable immunological tolerance to donor antigens. Furthermore, we observed an increase in the percentage of CD4+FoxP3+ regulatory T cells, which implies their role in tolerance induction.
C57BL/6 mouse, BALB/c mouse, C3H mouse and BALB/c.RAG2-/- mouse were purchased from The Jackson Laboratory (Sacramento, CA, USA). Mice (n=45) were bred and housed in a specific pathogen-free facility, Biomedical Center for Animal Resource Development at Seoul National University. Animal studies were conducted under protocols approved by Seoul National University institutional animal care and use committee (IACUC approval No. SNU-101228-3, 120306-3).
Recipient BALB/c mice were intraperitoneally injected with anti-asialo GM1 antibody (Wako Chemicals, Osaka, Japan) to deplete natural killer cells on the day before total body γ-ray irradiation (400-600 cGy). Bone marrow cells were obtained from the femur and tibia of C57BL/6 mouse. After tagging with mouse CD90.2 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany), T cells were sorted out by AutoMACS Separator (Miltenyi Biotec). Then, 2×107 of T cell-depleted C57BL/6 bone marrow cells were intravenously injected into the recipient BALB/c mouse. Four weeks later, establishment of mixed chimerism was assessed by flow cytometry of peripheral blood cells.
After incubation with Fc receptor blocking antibody, 2.4G2, peripheral blood or spleen cells were stained for 30 min on ice with a cocktail of antibodies for surface molecules. After washing twice with FACS buffer (PBS containing 0.5% BSA and 15.4 mM NaN3), cells were fixed and permeabilized with FoxP3 Fixation/Permeabilization buffer set (eBioscience, San Diego, CA, USA) according to the manufacturer's instructions. Then, intracellular FoxP3 was stained with specific antibody for 30 min on ice. After washing and re-suspending in FACS buffer, cells were acquired with a FACScan (BD Biosciences) and analyzed with CellQuest software (BD Biosciences).
Spleen cells were isolated by mechanical dissociation using fully frosted slides followed by lysis of red blood cells by RBC lysis buffer (Sigam-Aldrich, St. Louis, MO, USA). Prior to co-culturing, stimulator cells were inactivated by 20 Gy of γ-ray irradiation. Then, 5×105 stimulator cells and 5×105 responder cells were co-cultured in round-bottom 96-well plates for 24 hr, 48 hr or 72 hr at 37℃ in a 5% CO2 incubator. After 1 µCi of 3H-thymidine was added to each well, the cells were cultured for an extended period of 18 hr. Cells were harvested onto glass fiber filter, and 3H-thymidine incorporation was measured using a MicroBeta TriLux Microplate Scintillation Counter (Perkin Elmer, Wellesley, MA, USA).
Millipore multiscreen HA plates (Millipore, Bilerica, MA, USA) were coated with 3 µg/mL of anti-mouse IL-2, IL-4 or IFN-γ monoclonal antibodies overnight at 4℃. After washing, 5×105 fresh responder spleen cells were co-cultured with 5×105 stimulator spleen cells inactivated by 20 Gy of γ-ray irradiation at 37℃ in a 5% CO2 incubator. After 24 hr (IL-2 and IFN-γ) or 48 hr (IL-4) of culturing, cells were washed and incubated with 3 µg/mL of biotinylated anti-mouse IL-2, IL-4 or IFN-γ monoclonal antibodies overnight at 4℃. After washing, cells were incubated with avidin-alkaline phosphatase at 37℃ for 1 hr. Then, bound cytokines were visualized by incubation with BCIP/NBT (5-Bromo-4-chloro-3-indolyl phosphate/Nitro blue tetrazolium) substrate. The number of spots was counted using an AID ELISpot reader (AID, Strassberg, Germany).
Full-thickness tail skins obtained from donors were transplanted to graft beds on the left flank of the anesthetized recipient mice and covered with Vaseline gauze and Band-Aid (Johnson & Johnson, New Brunswick, NJ, USA). Bandages were removed after 7 days, and grafts were examined every 2 to 3 days for 3 weeks and weekly thereafter. The graft was scored as rejected when less than 10% of viable tissue remained.
We attempted to make mixed chimerism by using reduced doses of total body irradiation with a supplemental pre-depletion of NK cells. According to the schedule depicted in Fig. 1A, recipient BALB/c mice (H-2d) were depleted of NK cells with anti-asialo GM1 antibody injection. One day later, the recipients were exposed to various doses of total body irradiation. Then, T cell-depleted bone marrow cells obtained from C57BL/6 mice (H-2b) were intravenously injected into the recipients. In recipients which had received 450 cGy of irradiation, both C57BL/6 hematopoietic cells and BALB/c cells were detected (Fig. 1B). Although donor-origin hematopoietic cells could not be detected in the recipient that had received 400 cGy of irradiation, the recipient which had received 450-600 cGy of irradiation was able to induce mixed chimerism (Table 1). The recipients exhibited co-existence of hematopoietic cells of both recipient- and donor-origin, in addition to re-constitution of the immune compartment with diverse lymphocyte populations (Table 1). Since 450 cGy was sufficient to induce chimerism, this dose of γ-ray irradiation was used to establish mixed chimerism throughout this study.
To examine whether the chimeric recipient had rendered unresponsive to BM donor antigens, the allo-immune reactivity of the mixed chimera was evaluated. Proliferation of responder cells in response to allo-immune stimulus was assessed by 3H-thymidine incorporation in mixed leukocyte reaction. Although naïve BALB/c mouse exhibited potent allo-immune response to C57BL/6 antigens, the chimeric mouse cells exhibited significantly reduced allo-immune responses to C57BL/6 antigens (Fig. 2A). On the other hand, potent allo-immune response to 3rd party C3H mouse (H-2k) antigens was preserved in the mixed chimera (Fig. 2B). Next, allo-immune responses were measured at single cell levels with ELISpot assay. The level of IL-2, IFN-γ and IL-4-producing cells were significantly lower in the presence of C57BL/6 antigens compared to that in the presence of 3rd party C3H antigens (Fig. 2C). Although some allo-immune responses to BALB/c antigens existed, the level was lower than that elicited due to 3rd party C3H antigens (Fig. 2B, C).
To determine whether the low allo-immune responses result from donor-specific immunological tolerance or not, skin transplantation experiment was conducted. In addition, the transferability of tolerance was assessed by adoptive transfer. Control BALB/c.RAG2-/- mice which were adoptively transferred with naïve BALB/c spleen cells rejected C57BL/6 skin at day 14 after transplantation (Fig. 3A, B). On the other hand, when spleen cells obtained from the mixed chimeric mice were adoptively transferred into BALB/c.RAG2-/- mice, recipients did not reject C57BL/6 skin (Fig. 3A). Instead, black hair re-growth on C57BL/6 skin was observed, which indicated a successful acceptance (Fig. 3C). Since the recipient of chimeric cells rejected 3rd party C3H skin (Fig. 3D), the unresponsiveness was specific toward C57BL/6 skin. Collectively, the transferable donor-specific immunological tolerance was induced by mixed chimerism.
To find out how tolerance was induced, we analyzed immune cells in the mixed chimera and notable change was detected as a result. Percentage of CD4+FoxP3+ regulatory T (Treg) cells were increased in the mixed chimeric BALB/c mouse compared to that in naïve BALB/c mouse (Fig. 4A). This increase was attributed to the recipient-origin H-2d CD4+ T cells. The mixed chimera, which had received 450 cGy of irradiation, had Treg-enriched recipient-origin CD4+ T population as opposed to the recipient that had received 400 cGy of irradiation in which mixed chimera was not induced (Fig. 4B). This increase of BALB/c Treg cells suggests their role in the induction of immunological tolerance toward donor C57BL/6 antigens which happened in the immune compartment of the mixed chimera.
In this study, mixed chimerism could be established by a reduced dose of total body irradiation with supplemental depletion of NK cells. In the mixed chimera, reduced allo-immune response could be observed by reduced proliferation and cytokine production in the presence of bone marrow donor antigens. Then, the induction of transferable immunological tolerance was confirmed by adoptive transfer and subsequent acceptance of C57BL/6 skin graft. Finally, we speculated the role of regulatory T cells with the increase of them in the recipient compartment of the mixed chimera.
In the Fig. 2, the immune responses of chimeric recipients to BALB/c antigens were stronger than those to C57BL/6 antigens were. This result showed a close correlation with the larger portion of C57BL/6-derived hematopoietic cells in chimeric mice shown in Table 1. In line with this speculation, the frequency of T cells reactive to an antigen determines the magnitude of immune response towards that (14).
In the skin transplantation experiment shown in Fig. 3, after the adoptive transfer of spleen cells obtained from mixed chimeric mice into BALB/c.RAG2-/-mice, we observed that no C57BL/6-derived cells remained in the recipient (data not shown). This means that although a significant portion of spleen cells was C57BL/6-derived at the time they were adoptively transferred into the recipients, they became depleted while BALB/c-derived spleen cells remained at a steady level. Since NK cells are potent effector cells in the rejection of allogeneic bone marrow cells (15), we speculate that the transferred C57BL/6-derived spleen cells may be effectively removed by the action of NK cells of the BALB/c.RAG2-/- recipient. This indirectly indicates the significant contribution of NK cells to the rejection of allogeneic bone marrow cells, and emphasizes the necessity of NK-depletion prior to injecting donor cells. Therefore, we expect that depleting NK cells before the adoptive transfer under same experimental settings would allow for C57BL/6-derived spleen cells to remain in the recipient.
Although C57BL/6-derived spleen cells did not remain in recipients after the adoptive transfer in the above experiment, recipient mice still showed immune tolerance to the transplanted C57BL/6 skin (Fig. 3A). This result indicates that some populations of BALB/c-derived cells became tolerant to C57BL/6 donor antigens which may be explained by an increase in the percentage of CD4+FoxP3+ regulatory T cells (Fig. 4). We came up with two hypotheses about this phenomenon. One is that those C57BL/6-tolerant cells may be inducible regulatory T cells which were generated when both C57BL/6- and BALB/c-derived cells co-existed in the mixed chimera. The other is that although a small portion of inducible regulatory T cells developed, these cells led to the infectious tolerance (16) in BALB/c CD4+ cells, resulting in the increased frequency of regulatory T cells which are specific to C57BL/6 antigens. We expect that if we identify the antigen-specificity of these increased regulatory T cells, a considerable portion of them would be C57BL/6 antigen-specific. With those experiments verifying our hypotheses, we will be able to confirm the role of regulatory T cells in the induction of tolerance.
In this study, we have provided evidence that reprogramming the immune system of recipients by constructing BM mixed chimerism can induce donor-specific immune tolerance in allogeneic transplantation. Our data closely correspond with the insights of previous research concerning mixed chimerism, as it also contributes to the promising perspectives toward its application to clinics in minimizing the rejection. In addition, this study suggests that therapeutic usage of regulatory T cells may have supportive roles in the induction of transplantation tolerance by mixed chimerism.
Figures and Tables
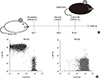 | Fig. 1Induction of mixed chimerism. (A) Recipient BALB/c mouse was given 2×107 T cell-depleted C57BL/6 bone marrow cells according to the depicted schedule. (B) 4 weeks later, the origin of hematopoietic cells present in recipients that had received distinct doses of total body irradiation was defined by expression of MHC (H-2Kb; C57BL/6, H-2Kd; BALB/c). Dot plots are gated in CD45+ hematopoietic cells. |
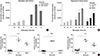 | Fig. 2Reduced allo-immune responses. 5×105 responder cells were co-cultured with 5×105 allogeneic stimulator cells. (A, B) Proliferation of responder cells was assessed by 3H-incorporation for 18 hr. (C) Number of cytokine-producing cells in response to allo-immune stimulus assessed by ELISpot assay is depicted with mean and standard deviation. Each dot represents an individual mouse. *P < 0.001. |
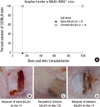 | Fig. 3Transferable tolerance to bone marrow donor. BALB/c.RAG2-/-mice were adoptively transferred with spleen cells of the mixed chimeric mice or of naïve BALB/c mice. Then, C57BL/6, BALB/c or C3H full-thickness tail skins were transplanted on left flanks of the recipients. (A) Percent survival of transplanted C57BL/6 skin is depicted. (B-D) Photographs of representative recipients' skins on indicated days are displayed. In the photographs, the left parts are the BALB/c skin, and the right parts are C57BL/6 skin (B, C) or C3H skin (D). |
 | Fig. 4Increased percentage of Treg cells in recipient-compartment of the mixed chimera. Ten weeks after the bone marrow transplantation, spleen cells obtained from the mixed chimera which had received 450 cGy (upper panels), or 400 cGy (lower panel of B) of irradiation or naïve BALB/c mouse (lower panel of A) were analyzed. Dot plots were gated in CD3+ cells. |
Notes
References
1. Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012; 12:417–430.
2. Zelenika D, Adams E, Humm S, Lin CY, Waldmann H, Cobbold SP. The role of CD4+ T-cell subsets in determining transplantation rejection or tolerance. Immunol Rev. 2001; 182:164–179.
3. Cornell LD, Smith RN, Colvin RB. Kidney transplantation: mechanisms of rejection and acceptance. Annu Rev Pathol. 2008; 3:189–220.
4. Wekerle T, Sykes M. Mixed chimerism as an approach for the induction of transplantation tolerance. Transplantation. 1999; 68:459–467.
5. Sachs DH. Tolerance: of mice and men. J Clin Invest. 2003; 111:1819–1821.
6. Pilat N, Wekerle T. Transplantation tolerance through mixed chimerism. Nat Rev Nephrol. 2010; 6:594–605.
7. Pilat N, Hock K, Wekerle T. Mixed chimerism through donor bone marrow transplantation: a tolerogenic cell therapy for application in organ transplantation. Curr Opin Organ Transplant. 2012; 17:63–70.
8. Park M, Koh KN, Seo JJ, Im HJ. Clinical implications of chimerism after allogeneic hematopoietic stem cell transplantation in children with non-malignant diseases. Korean J Hematol. 2011; 46:258–264.
9. Salama AD, Remuzzi G, Harmon WE, Sayegh MH. Challenges to achieving clinical transplantation tolerance. J Clin Invest. 2001; 108:943–948.
10. Ildstad ST, Wren SM, Bluestone JA, Barbieri SA, Sachs DH. Characterization of mixed allogeneic chimeras: immunocompetence, in vitro reactivity, and genetic specificity of tolerance. J Exp Med. 1985; 162:231–244.
11. Murphy WJ, Kumar V, Bennett M. Acute rejection of murine bone marrow allografts by natural killer cells and T cells: differences in kinetics and target antigens recognized. J Exp Med. 1987; 166:1499–1509.
12. Cho SG, Shuto Y, Soda Y, Nakazaki Y, Izawa K, Uchimaru K, Takahashi S, Tani K, Tojo A, Asano S. Anti-NK cell treatment induces stable mixed chimerism in MHC-mismatched, T cell-depleted, nonmyeloablative bone marrow transplantation. Exp Hematol. 2004; 32:1246–1254.
13. Kasai M, Yoneda T, Habu S, Maruyama Y, Okumura K, Tokunaga T. In vivo effect of anti-asialo GM1 antibody on natural killer activity. Nature. 1981; 291:334–335.
14. Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007; 27:203–213.
15. Kean LS, Hamby K, Koehn B, Lee E, Coley S, Stempora L, Adams AB, Heiss E, Pearson TC, Larsen CP. NK cells mediate costimulation blockade-resistant rejection of allogeneic stem cells during nonmyeloablative transplantation. Am J Transplant. 2006; 6:292–304.
16. Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, Waldmann H. "Infectious" transplantation tolerance. Science. 1993; 259:974–977.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download