Abstract
Sporadic spastic paraplegia (SSP) and hereditary spastic paraplegia (HSP) belong to a clinical and genetically heterogeneous group of disorders characterized by progressive spasticity and weakness in the lower extremities. The symptoms are associated with pyramidal tract dysfunction and degeneration of the corticospinal tracts. Parkinsonism is uncommon in SSP/HSP patients. However, both disorders are associated with damage to the nigrostriatal dopaminergic system. In the present study, the clinical features of patients with SSP/HSP were investigated, and nigrostriatal dopaminergic binding potential was assessed using dopamine transporter (DAT) single-photon emission computer tomography (SPECT). Nine patients with spastic paraplegia participated in the present study. The subjects underwent DAT SPECT using the agent [2-[[2-[[[3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3,2,1]oct-2-yl]methyl](2-mercaptoethyl)amino]ethyl]amino]ethanethiolato (3-)-N2,N20,S2,S20]oxo-[IR-(exo-exo)])-[99mTc]technetium ([99mTc]TRODAT-1). The [99mTc]TRODAT-1 SPECT images of five patients appeared normal, whereas the images of four patients revealed reduced striatal ligand uptake. Among the four patients with reduced uptake, two had parkinsonism, and one exhibited periodic limb movements and restless leg syndrome. Our DAT SPECT imaging study shows that reduced DAT density may be observed in patients with parkinsonism. The results of the present study offer an explanation for the spectrum of spastic paraplegia symptoms and the progression of the disorder.
Hereditary spastic paraplegia (HSP) is a clinically and genetically heterogeneous group of neurodegenerative disorders that include the clinical features of progressive spasticity and lower-limb weakness (1). Recent advances, including the identification of new genes and loci related to HSP, have greatly improved the diagnosis of HSP. More than 45 spastic paraplegia genes (SPG) and 20 causative genes have been identified. Moreover, multiple modes of genetic inheritance have been reported, including autosomal dominant, autosomal recessive, X-linked, and sporadic spastic paraplegia (SSP) (2).
As a result of the genetic variability observed in patients with HSP, the clinical spectrum of HSP subtypes has widened. Traditionally, two subtypes were distinguished (i.e., pure and complicated) based on the presence (complicated form) or absence (pure form) of accompanying clinical features in addition to spastic paraparesis (1). Although the clinical classification of spastic paraplegia is straightforward, the diagnosis of HSP is based on phenotype and thus remains difficult for clinicians. The clinical symptoms of the complicated subtype of HSP may include cognitive impairment, epilepsy, neuropathy, ataxia, or extrapyramidal symptoms. These symptoms often overlap with other neurodegenerative diseases. Among the complicated subtypes, spastic paraplegia with parkinsonism can present with HSP as well as with other diseases such as spinocerebellar ataxia (SCA) and dopa-response parkinsonism (3-9). SCA type 3 (SCA3; Machado-Joseph disease) is an autosomal dominant disease with various clinical manifestations. SCA3 subtype V cases are often misdiagnosed as HSP because of the presence of spastic paraparesis (3-5, 10, 11). A few reported cases had autosomal recessive HSP with a thin corpus callosum; these cases have been associated with mutations of SPG11, and they typically present with unusual early-onset parkinsonism (7, 8, 12).
The cause of parkinsonism in HSP and SSP is unknown. Dopamine transporter (DAT) single photon emission computed tomography (SPECT) with 123I-ioflupane revealed that patients with the SPG11 mutation and parkinsonism exhibited abnormal DAT binding potential (7, 12). Moreover, Lee and colleagues reported that Drosophila bearing a null mutation in a SPG3A (atlastin-1) homolog showed age-related degeneration of dopaminergic neurons (13). Another study using DAT positron emission tomography (PET) with 11C-dihydrotetrabenazine, however, revealed that patients with SPG3A displayed normal binding potential (14).
Therefore, the aim of the present study was to examine the clinical symptoms of patients with spastic paraplegia with and without parkinsonism. Specifically, DAT SPECT was performed using [2-[[2-[[[3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3,2,1]oct-2-yl]methyl](2-mercaptoethyl)amino]ethyl]amino]ethanethiolato (3-)-N2,N20,S2,S20]oxo-[IR-(exo-exo)])-[99mTc]technetium ([99mTc]TRODAT-1) to determine whether the nigrostriatal dopaminergic system is altered in patients with variable clinical features.
All patients were clinically diagnosed with SSP or HSP at the Department of Neurology of Seoul National University Hospital, and participated in this study over a 5-yr period. The diagnosis of HSP was based on the criteria adapted from Harding (1). All of the patients displayed key diagnostic clinical features, including lower-limb spasticity and pyramidal weakness, as well as hyperreflexia and extensor plantar responses. The differential diagnosis of HSP included other diseases that cause slowly progressive paraplegia, (i.e., structural spinal cord abnormalities) including degenerative diseases such as amyotrophic lateral sclerosis, leukodystrophies, and metabolic and infectious disorders. All patients underwent brain and whole-spine MRI. Genetic studies including HSP (SPG3, 4) and SCA (types 1, 2, 3, 4, 6, 7, 17), as well as laboratory studies were performed selectively according to the participant's clinical features.
Detailed procedures of [99mTc]-TRODAT-1 SPECT were performed as previously described (15).
Nine native Korean patients with the clinical symptoms of SSP/HSP were recruited for the present study. Three (patients 1-3) had a family history of spastic paraplegia, and six (patients 4-9) had no such family history. One of the patients (patient 4) displayed the pure subtype, and the others exhibited complicated spastic paraplegia. Among these, two of the cases (patients 7 and 8) had parkinsonism.
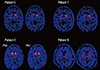
The DAT densities of five patients appeared normal (patients 1-5). Four patients (patients 6-9) displayed reduced DAT uptake (three patients displayed a bilateral uptake reduction, and one patient displayed a unilateral reduction) (Fig. 1, 2). Clinical information provided below and the characteristics of the nine patients are summarized in Table 1.
Patients 1 and 2 are siblings. The brother was healthy and enrolled in military service in his early 20s. At the age of 32 yr, he started to suffer from gait disturbance. This symptom gradually progressed and he experienced dysarthria and weakness in his left leg 5 yr later. Upon hospital admission at the age of 53, the patient showed dysarthric speech and dysmetria. Deep tendon reflexes (DTRs) were enhanced in both legs, his gait was spastic, he could walk independently. The patient showed mild dysmetria in his left arm, however the upper extremities were not spastic and he could use chopsticks. His parents and elder brothers were healthy and without any neurological deficits, and his parents were not consanguineous. He is now 58 yr old, his spastic gait has deteriorated slightly, and he walks with a cane and often falls.
The sister was healthy until the age of 32 yr, when she noticed an unsteady gait. Gait disturbance progressed very slowly. Twelve years later, she developed dysarthria and urinary urgency. At age 47, she was admitted to the hospital with her brother. Neurologic examination revealed dysarthria and mild spasticity of the legs with enhanced DTRs. Sustained ankle clonus was noted. Ocular movement and cerebellar function were normal. The patient had no extrapyramidal signs or cognitive dysfunctions. At the last follow-up (at age 52), her spastic gait was aggravated, however, she was still able to walk independently.
This 50-yr-old male was admitted because of a progressive gait disturbance that developed at age 40. Five years later, he became wheelchair-bound. Neurological examination showed severe spasticity in both legs, with marked enhanced tendon reflexes and extensor plantar responses. Muscle strength was decreased to grade 4 (Medical Research Council) in both lower extremities. The patient showed intermittent myoclonus in both legs that started 10 yr prior. He did not have cerebellar dysfunction. Eye movement examination revealed square wave jerk. He had a cystostomy because of urinary retention. Genetic studies for SCA types 1, 2, 3, 6, and 7 and SPG4 were all negative. His mother exhibited spastic gait in her 40s; however her gait disturbance were progressed minimally, and she could walk independently for 30 yr. Other family members were healthy and without any neurological problems.
A 51-yr-old male was diagnosed with SSP. When he was 33 yr old, he experienced right leg weakness and gait disturbance that progressed very slowly. On neurological examination, he presented with spasticity and muscular strength grade 4 in the lower limbs. The patient had increased DTRs in the lower limbs, with bilateral extensor plantar responses and ankle clonus. He is now 56 yr old, and his neurological signs are similar to the initial findings.
This 44-yr-old female presented with a 1-yr history of progressive weakness in the lower limbs. Her gait was spastic and ataxic in nature. To ascertain whether she had a genetic disease, she was evaluated for SCA types 1, 2, 3, 5, 7, and 8 and all were normal. She presented with normal cranial nerves, and spasticity muscular strength grade 4 in the lower limbs. Her gait became aggravated, and she is now at the age of 49 yr wheelchair-bound.
This 59-yr-old male was healthy until the age of 49 yr, when he experienced unsteadiness and gait imbalance. He complained of sensory paresthesia in his feet and orthostatic dizziness. Neurological examination revealed spastic ataxia and postural instability. The patient showed spasticity of the legs with motor weakness. He had autonomic dysfunction on breath test (expiration/inspiration ratio=1.04, normal range>1.08), and the other autonomic functions were normal. He was taking amantadine (200 mg per day) without effect. Orthostatic dizziness disappeared during the follow-up period. Genetic evaluation for SCA types 1, 2, 3, 6, 7, and 17, SPG 4, and Friedreich ataxia (frataxin) were all negative. The patient is now 63 yr old and shows spastic gait, leg weakness, and paresthesia. Orthostatic dizziness, and other dysautonomic symptoms were not present.
A 64-yr-old male presented with a progressive spastic gait and pain in both legs starting 4 yr prior. On neurological examination, he revealed spasticity with brisk DTRs and ankle clonus. He exhibited postural hand tremor and rigidity, primarily on the right side, as well as subtle bradykinesia on rapid alternating movement. On cranial nerve function test, the patient had sensory neural hearing loss and showed head-shaking nystagmus suggestive of left vestibulopathy. His arm motor function was normal, and the motor strength of his legs was mildly decreased (Rt hip 3, Lt hip 4+, Rt knee 4, Lt knee 4+). He complained of urinary hesitancy and difficulty on initial examination; however, approximately 2 months after the initial examination, voiding symptoms disappeared spontaneously. Other autonomic dysfunctions were not observed. He was taking anti-parkinsonian medications for 13 months (last medications were levodopa 600 mg, pramipexole 1.5 mg, amantadine 100 mg per day); however the medications were discontinued due to ineffectiveness and continuous aggravation. SPG4 was normal and SCA genetic tests were not performed. On his last visit (at the age 67 yr), neurological symptoms were worse. He was wheelchair-bound and showed severe dysarthria, dysphagia, and generalized myalgia. He died at the age of 68 yr.
This 34-yr-old female visited the hospital because of spastic gait and weakness in the right leg. She had the symptoms for 2 yr, and they slowly became worse. The DTRs were markedly exaggerated with positive Babinski signs. The motor strength of her legs was grade 4. She had right-beating nystagmus on virbration. The patient had the following parkinsonian features: dysarthria, hypomimia, hypophonia, postural tremor, rigidity, and bradykinesia (Unified Parkinson's Disease Rating Scale, 22). She complained of occasional orthostatic dizziness; however, orthostatic blood pressure, urinary function tests were normal. The genetic analysis excluded SPG3, 4, and SCA 2, 3, and 17. After taking anti-parkinsonian medications (levodopa/carbidopa 1200 mg, pramipexole 1.5 mg per day), tremor, bradykinesia, and rigidity were partially improved. During the 5-yr follow-up period, she experienced intermittent dizziness that was not troublesome, and neurological symptoms were slightly aggravated.
This 73-yr-old male showed gait disturbance at age 71. On walking, he showed a typical spastic gait with postural abnormality. The patient had spastic paraparesis with bilateral Babinski signs and brisk DTRs. After reviewing his sleep pattern, we concluded he had rapid eye movement (REM) sleep behavior disorder (RBD) and periodic limb movement disorder (PLMD). He was diagnosed approximately 8 yr prior with benign prostate hypertrophy, and was suffering from voiding symptoms including frequencyuria and urgency. The genetic tests, including Friedreich ataxia, SPG4, and SCA types 2 and 3, were normal.
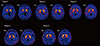
Four of the patients (patients 6-9) in the present study displayed reduced DAT uptake on SPECT imaging, which suggests a loss of dopaminergic neurons. Among them, patients 7 and 8 had parkinsonism that included tremors, bradykinesia, and rigidity. Patients 6 and 9 both displayed reduced DAT density but no clinical features of parkinsonism.
The definitive diagnoses of these 4 patients were unclear. Multiple system atrophy (MSA) or SCA could not be ruled out. MSA is characterized by varying degrees of parkinsonism, cerebellar ataxia, autonomic failure, and urogenital dysfunction. Generally, autonomic dysfunction is a major feature of MSA. DAT imaging studies have shown the ability to detect loss of striatal dopamine transporters in patients with presynaptic parkinsonism such as PD or MSA (16). However, the clinical manifestations of our patients with decreased DAT uptake were somewhat different from those associated with MSA or SCA. Patient 6 experienced autonomic dysfunction, which is a major symptom of MSA, but it was transient and not severe. Genetic studies for SCA 1, 2, 3, 6, 7, and 17 were negative. Patients 7 and 8 had spastic paraplegia with parkinsonism. Patient 7 did not show autonomic failure in any of the laboratory evaluations except the respiratory test, which produced a subclinical finding. Additionally, cerebellar atrophy was not obvious on brain MRI. The patient did not have the SCA genetic test performed, which was a limitation of this study. Patient 8 was relatively young at onset and did not show autonomic or cerebellar symptoms. During the 5-yr follow-up period, her neurological symptoms progressed very slowly, and SCA tests (2, 3, 17) were negative. Patient 9 had REM RBD and PLMD, which may be associated with reduced striatal uptake, regardless of clinical parkinsonism (17). Previous studies have noted that the idiopathic form of RBD may be a pre-motor feature of an evolving synucleinopathy. Furthermore, a reduction in striatal tracer uptake indicates dopaminergic damage (18-20). RBD and PLMD could have influenced the nigrostriatal pathway in patient 9. Perhaps the patients with decreased DAT uptake had spastic paraplegia, comorbid with neurological disease that included presynaptic dopaminergic degeneration. However, even in patients with parkinsonism, levodopa was ineffective (patient 7) or partially effective (patient 8). During the 5-yr follow-up period, the parkinsonian features did not progress, which reduced the possibility of comorbid presynaptic dopaminergic degenerative disorders. In addition, SSP/HSP might be related to the loss of striatal dopamine transporters. During the past 30 yr, advances in assessing genetic disorders have resulted in striking improvements; however, the clinical boundaries of HSP still remain obscure. Although extensive diagnostic testing was conducted, other etiologies could not be ruled out, which is a limitation of the present study.
HSP is associated not only with a broad spectrum of clinical and genetic features but also with pathological multisystem degeneration. According to previous reports, the core neuropathological features of pure HSP consist of damage to the longest descending and ascending tracts of the spinal cord and degeneration in layer V of the primary motor cortex, cerebellum, and spinocerebellar tracts. Patients with complicated HSP display widespread degenerative changes in the cerebellum, cerebral cortex, thalamus, and brainstem (21, 22). Kuru and colleagues (23) reported neurodegeneration in the corticospinal tract, thalamus, cerebral white matter, substantia nigra, and anterior horn and in the posterior column of the spinal cord in a spastic paraplegia patient with a thin corpus callosum. Postmortem findings have provided some interpretation of the various clinical features and indicated the extent of brain degeneration that may also be present in patients with HSP.
The pathophysiology of HSP is not fully understood; however, genetic mechanisms appear to underlie the multiple defects at cellular sites that lead to axon disturbance. At least 20 genes have been implicated in HSP. The genes are involved in cell adhesion, myelination, mitochondrial function, and neuronal trafficking. This suggests that deficits in neuronal molecules and organelles may be responsible for the variable phenotypes associated with HSP (24, 25). Lee and colleagues reported that a Drosophila HSP model bearing a null mutation in atl exhibited paralysis following mechanical shock, premature mortality, and age-related degeneration of dopaminergic neurons. These phenotypes were rescued by targeted expression of atl in dopaminergic neurons or by administering Levodopa or another dopamine receptor agonist (13). Observations of dopaminergic neuron vulnerability in HSP patients raise the possibility that degeneration of central neurons may contribute to the phenotype of HSP.
The present DAT SPECT imaging study provides further clarification of the features associated with dopaminergic neuronal integrity that may be present in patients with SSP/HSP. Our results show that reduced DAT density may be observed in patients with spastic paraplegia with parkinsonism. Moreover, the molecular imaging techniques used in the present study may help in clarifying the pathomechanism of SSP/HSP as well as provide evidence for the effectiveness of treatments.
Figures and Tables
Fig. 2
[99mTc]TRODAT-1 SPECT: Diminished DAT uptake imaging results (patients 6-9). Patients 7 and 8 had parkinsonism, whereas patient 9 showed bradykinesia, RBD, and PLMD.
Notes
References
1. Harding AE. Classification of the hereditary ataxias and paraplegias. Lancet. 1983; 1:1151–1155.
2. Dion PA, Daoud H, Rouleau GA. Genetics of motor neuron disorders: new insights into pathogenic mechanisms. Nat Rev Genet. 2009; 10:769–782.
3. Sakai T, Kawakami H. Machado-Joseph disease: a proposal of spastic paraplegic subtype. Neurology. 1996; 46:846–847.
4. Kaneko A, Narabayashi Y, Itokawa K, Nakazato Y, Hosokawa T, Iwasaki S, Ohno R, Hamaguchi K, Ikeda M, Nomura M. A case of Machado-Joseph disease presenting with spastic paraparesis. J Neurol Neurosurg Psychiatry. 1997; 62:542–543.
5. Teive HA, Iwamoto FM, Camargo CH, Lopes-Cendes I, Werneck LC. Machado-Joseph disease versus hereditary spastic paraplegia: case report. Arq Neuropsiquiatr. 2001; 59:809–811.
6. Micheli F, Cersósimo MG, Zúñiga Ramírez C. Hereditary spastic paraplegia associated with dopa-responsive parkinsonism. Mov Disord. 2006; 21:716–717.
7. Guidubaldi A, Piano C, Santorelli FM, Silvestri G, Petracca M, Tessa A, Bentivoglio AR. Novel mutations in SPG11 cause hereditary spastic paraplegia associated with early-onset levodopa-responsive Parkinsonism. Mov Disord. 2011; 26:553–556.
8. Kang SY, Lee MH, Lee SK, Sohn YH. Levodopa-responsive parkinsonism in hereditary spastic paraplegia with thin corpus callosum. Parkinsonism Relat Disord. 2004; 10:425–427.
9. Dick AP, Stevenson CJ. Hereditary spastic paraplegia; report of a family with associated extrapyramidal signs. Lancet. 1953; 1:921–923.
10. Hedera P. Hereditary spastic paraplegia or spinocerebellar ataxia? not always as easy as it seems. Eur J Neurol. 2009; 16:887–888.
11. Wang YG, Du J, Wang JL, Chen J, Chen C, Luo YY, Xiao ZQ, Jiang H, Yan XX, Xia K, et al. Six cases of SCA3/MJD patients that mimic hereditary spastic paraplegia in clinic. J Neurol Sci. 2009; 285:121–124.
12. Anheim M, Lagier-Tourenne C, Stevanin G, Fleury M, Durr A, Namer IJ, Denora P, Brice A, Mandel JL, Koenig M, et al. SPG11 spastic paraplegia: a new cause of juvenile parkinsonism. J Neurol. 2009; 256:104–108.
13. Lee Y, Paik D, Bang S, Kang J, Chun B, Lee S, Bae E, Chung J, Kim J. Loss of spastic paraplegia gene atlastin induces age-dependent death of dopaminergic neurons in Drosophila. Neurobiol Aging. 2008; 29:84–94.
14. Albin RL, Koeppe RA, Rainier S, Fink JK. Normal dopaminergic nigrostriatal innervation in SPG3A hereditary spastic paraplegia. J Neurogenet. 2008; 22:289–294.
15. Kim JY, Kim SY, Kim JM, Kim YK, Yoon KY, Kim JY, Lee BC, Kim JS, Paek SH, Park SS, et al. Spinocerebellar ataxia type 17 mutation as a causative and susceptibility gene in parkinsonism. Neurology. 2009; 72:1385–1389.
16. Booij J, Speelman JD, Horstink MW, Wolters EC. The clinical benefit of imaging striatal dopamine transporters with [123I]FP-CIT SPET in differentiating patients with presynaptic parkinsonism from those with other forms of parkinsonism. Eur J Nucl Med. 2001; 28:266–272.
17. Iranzo A, Valldeoriola F, Lomeña F, Molinuevo JL, Serradell M, Salamero M, Cot A, Ros D, Pavía J, Santamaria J, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011; 10:797–805.
18. Albin RL, Koeppe RA, Chervin RD, Consens FB, Wernette K, Frey KA, Aldrich MS. Decreased striatal dopaminergic innervation in REM sleep behavior disorder. Neurology. 2000; 55:1410–1412.
19. Eisensehr I, Linke R, Noachtar S, Schwarz J, Gildehaus FJ, Tatsch K. Reduced striatal dopamine transporters in idiopathic rapid eye movement sleep behaviour disorder: comparison with Parkinson's disease and controls. Brain. 2000; 123:1155–1160.
20. Stiasny-Kolster K, Doerr Y, Möller JC, Höffken H, Behr TM, Oertel WH, Mayer G. Combination of 'idiopathic' REM sleep behaviour disorder and olfactory dysfunction as possible indicator for alpha-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain. 2005; 128:126–137.
21. Wharton SB, McDermott CJ, Grierson AJ, Wood JD, Gelsthorpe C, Ince PG, Shaw PJ. The cellular and molecular pathology of the motor system in hereditary spastic paraparesis due to mutation of the spastin gene. J Neuropathol Exp Neurol. 2003; 62:1166–1177.
22. Seidel K, De Vos R, Derksen L, Bauer P, Riess O, den Dunnen W, Deller T, Hageman G, Rüb U. Widespread thalamic and cerebellar degeneration in a patient with a complicated hereditary spastic paraplegia (HSP). Ann Anat. 2009; 191:203–211.
23. Kuru S, Sakai M, Konagaya M, Yoshida M, Hashizume Y. Autopsy case of hereditary spastic paraplegia with thin corpus callosum showing severe gliosis in the cerebral white matter. Neuropathology. 2005; 25:346–352.
24. Trotta N, Orso G, Rossetto MG, Daga A, Broadie K. The hereditary spastic paraplegia gene, spastin, regulates microtubule stability to modulate synaptic structure and function. Curr Biol. 2004; 14:1135–1147.
25. Salinas S, Carazo-Salas RE, Proukakis C, Schiavo G, Warner TT. Spastin and microtubules: functions in health and disease. J Neurosci Res. 2007; 85:2778–2782.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download