Abstract
Hypersensitivity to mosquito bites (HMB) is a rare disease characterized by intense skin reactions such as bulla and necrotic ulcerations at bite sites, accompanied by general symptoms such as high-grade fever and malaise occurred after mosquito bites. It has been suggested that HMB is associated with chronic Epstein-Barr virus (EBV) infection and natural killer (NK) cell leukemia/lymphoma. We describe here a Korean child who presented with 3-yr history of HMB without natural killer cell lymphocytosis. He has been ill for 6 yr with HMB. Close observation and examination for the development of lymphoproliferative status or hematologic malignant disorders is needed.
Hypersensitivity to mosquito bites (HMB) is a rare disease characterized by severe skin reactions such as bulla and necrotic ulcerations at bite sites, accompanied by general symptoms such as high-grade fever and lymphadenopathy occurred after mosquito bites. It has been suggested that HMB is associated with chronic Epstein-Barr virus (EBV) infection and natural killer (NK) cell leukemia/lymphoma, so was named "HMB-EBV-NK disease" (1).
Few clear cases of HMB have been reported in Korea (2-4) and the prevalence of HMB seems to be far lower than in Japan, where about 50 cases accumulated in 2001 (1). We describe a Korean child who presented with 3-yr history of recurrent skin reactions and high fever after mosquito bite without peripheral NK cell lymphocytosis.
A 6-yr-old boy was admitted to our hospital for management and evaluation of recurrent skin reactions developed after mosquito bites in July 2010. He had high-grade fever up to 40℃ and severe skin lesions developing after mosquito bites. For three consecutive summer seasons (2007-2009), he had been admitted to the hospital or medicated at outpatient clinic due to an erythematous swelling and bulla formation at bite site with fever, regional lymphadenopathy or phlebitis after mosquito bites. He was diagnosed with cellulitis for each episode since then. These lesions evolved into necrotic ulcers and healed with residual scarring. On physical examinations at admission, erythematous swelling with a central bulla was found on his right ankle (Fig. 1A). His liver and spleen were not palpable. Lung sound was clear and chest X-ray revealed no infiltrations.
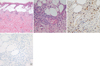
Laboratory tests showed white blood cells 6,160/mL with 28.6% of lymphocytes and 0.1% of eosinophils, normal ranges of hemoglobin, liver function test, and lactate dehydrogenase. Urinalysis was normal. IgE by PRIST was above 5,000 kIU/L with normal range of IgG, A, M levels. Lymphocyte subset analysis demonstrated normal percentage of CD3 (77.4%), CD4 (33.1%), CD8 (41.6%), NK cell (8.4%), and CD19 (15.2%). IgM for viral capsid antigen (VCA) and IgM for anti-early antigen (EA) DR to EBV were negative. However, anti-VCA IgG (5.5) and anti-EBNA IgG were positive (15.5). Skin biopsy from the lesion showed intraepidermal vesicles with interstitial and perivascular infiltration of lymphocytes, neutrophils, eosinophils and histiocytes (Fig. 2A, B). EBV encoded RNA (EBER) in situ hybridization was positive (Fig. 2C) and immunohistochemical staining with NK cell marker (CD56) of biopsy specimen was negative (Fig. 2D). We did not perform bone marrow biopsy because his laboratory findings and physical examinations did not suggest the lymphoproliferative state or malignancy.
He suffered from iridocyclitis two months after the diagnosis and was improved with medication of ophthalmology department. During the next summer season, he was admitted twice because of severe skin reactions and fever after mosquito bite (Fig. 1B, C). Laboratory tests at that time showed still a normal percentage of NK cells. EBV-DNA viral load by PCR analysis was low and not in the active state (38/µL DNA). Now, he has undergone follow-up observations for two years after diagnosis of HMB, having been ill for a total of six years from the beginning of the disease without the evidence of development of lymphoproliferative status or hematologic malignant disorders.
HMB is known to be one of the symptoms of NK-cell type of chronic active EBV (CAEBV) infection (5-7). The NK-cell type of CAEBV infection is characterized by higher EBV DNA loads, high titers of IgE, hypersensitivity to mosquito bite and better prognosis than that of T-cell type (5-7). Unlike the classification of NK/T-cell type from the previous studies in Japan, B-cell type is dominant form of CAEBV and HBM is one of the symptoms of NK-cell type, similarly, in USA (8). In the present case, the patient had high IgE level.
His infectious condition has not progressed to CAEBV infection so far. Viral load exceeding 102.5 copies/µg of DNA is suggested as a diagnostic criterion for CAEBV (5). Monitoring of EBV viral load is useful for evaluation of disease activity in patients suffering from HMB (9). In Japan, spontaneous improvement was observed in some patients with HMB (1). In our case, although the skin biopsy at bite site was revealed EBER-positive by in situ hybridization, EBV-DNA viral load was low.
To our knowledge, three cases of HMB were reported in Korea. The patients were a 19-yr-old male with NK cell-derived large granular lymphocytosis (2), a 20-yr-old male with NK cell lymphoma who had a HMB history from childhood (3), and a 5-yr-old boy with NK cell lymphocytosis, who was diagnosed with hemophagocytic lymphohistiocytosis (HLH) developed after 2.5 yr of HMB (4). This is the second case of childhood HMB in Korea. In contrast to previously reported cases (2, 4), the present patient had a normal NK cell percentage. We could not detect any findings of hematologic malignancies in him as yet. NK/T-cell lymphoma was recently reported from China developed after a history of two years earlier of HMB in a 9-yr-old girl (10).
Pathogenic mechanisms linking HMB, EBV infection, and NK cell oncogenesis was suggested by Asada (11). CD4+ T cells stimulated by mosquito salivary gland extracts could induce reactivation of latent EBV infection in NK cells and have an important role on the intense skin reaction to mosquito bite. Also, mosquito bites can induce EBV-oncogene LMP1 in NK cells via mosquito antigen-specific CD4+ T cells, which is involved in the oncogenesis of NK cells.
Hematopoietic stem cell transplantation (HSCT) has been performed as a curative treatment of CAEBV infection (5, 8, 12). Although the outcome of HSCT with reduced-intensity conditioning for CAEBV infection was reported to be promising (13), there is no clear evidence of treating aggressively a simple HMB without the evidence of CAEBV infection or EBV reactivation.
In conclusion, we present a case of 6-yr duration of HMB without NK cell lymphocytosis. Close observation and examination for the development of HLH or lymphoid malignancy is needed. In the future, therapies such as chemotherapy and HSCT should be considered upon evidence of him progressing to CAEBV infection.
Figures and Tables
Fig. 1
Gross appearance of the skin lesion. (A) Erythematous swelling with a central bulla. (B) Another bullous skin lesion developed 1 yr after diagnosis of HMB. (C) Eschar formation from a bulla at a mosquito bite site.

Fig. 2
Pathologic findings of the skin lesion. (A: H&E, × 100, B: × 400). Histopathologic examination of the skin biopsy specimen showed intraepidermal vesicles with interstitial and perivascular infiltration of lymphocytes, neutrophils, eosinophils and histiocytes. (C) In situ hybridization analysis for EBER was positive (dark brown color, × 400). (D) Immunohistochemical staining with CD56 was negative (× 400).
References
1. Tokura Y, Ishihara S, Tagawa S, Seo N, Ohshima K, Takigawa M. Hypersensitivity to mosquito bites as the primary clinical manifestation of a juvenile type of Epstein-Barr virus-associated natural killer cell leukemia/lymphoma. J Am Acad Dermatol. 2001. 45:569–578.
2. Chung JS, Shin HJ, Lee EY, Cho GJ. Hypersensitivity to mosquito bites associated with natural killer cell-derived large granular lymphocyte lymphocytosis: a case report in Korea. Korean J Intern Med. 2003. 18:50–52.
3. Cho JH, Kim HS, Ko YH, Park CS. Epstein-Barr virus infected natural killer cell lymphoma in a patient with hypersensitivity to mosquito bite. J Infect. 2006. 52:e173–e176.
4. Roh EJ, Chung EH, Chang YP, Myoung NH, Jee YK, Seo M, Kang JH. A case of hypersensitivity to mosquito bite associated with Epstein-barr viral infection and natural killer cell lymphocytosis. J Korean Med Sci. 2010. 25:321–323.
5. Kimura H, Hoshino Y, Kanegane H, Tsuge I, Okamura T, Kawa K, Morishima T. Clinical and virologic characteristics of chronic active Epstein-Barr virus infection. Blood. 2001. 98:280–286.
6. Pacheco SE, Gottschalk SM, Gresik MV, Dishop MK, Okmaura T, Mc-Cormick TG. Chronic active Epstein-Barr virus infection of natural killer cells presenting as severe skin reaction to mosquito bites. J Allergy Clin Immunol. 2005. 116:470–472.
7. Kimura H, Hoshino Y, Hara S, Sugaya N, Kawada J, Shibata Y, Kojima S, Nagasaka T, Kuzushima K, Morishima T. Differences between T cell-type and natural killer cell-type chronic active Epstein-Barr virus infection. J Infect Dis. 2005. 191:531–539.
8. Cohen JI, Jaffe ES, Dale JK, Pittaluga S, Heslop HE, Rooney CM, Gottschalk S, Bollard CM, Rao VK, Marques A, et al. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States. Blood. 2011. 117:5835–5849.
9. Satoh M, Oyama N, Akiba H, Ohtsuka M, Iwatsuki K, Kaneko F. Hypersensitivity to mosquito bites with natural-killer cell lymphocytosis: the possible implication of Epstein-Barr virus reactivation. Eur J Dermatol. 2002. 12:381–384.
10. Zhang Z, Shi Q, An X, Ma H, Zhou H, Ma J, Zhou X. NK/T-cell lymphoma in a child with hypersensitivity to mosquito bites. J Pediatr Hematol Oncol. 2009. 31:855–857.
11. Asada H. Hypersensitivity to mosquito bites: a unique pathogenic mechanism linking Epstein-Barr virus infection, allergy and oncogenesis. J Dermatol Sci. 2007. 45:153–160.
12. Kimura H, Ito Y, Kawabe S, Gotoh K, Takahashi Y, Kojima S, Naoe T, Esaki S, Kikuta A, Sawada A, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. 2012. 119:673–686.
13. Kawa K, Sawada A, Sato M, Okamura T, Sakata N, Kondo O, Kimoto T, Yamada K, Tokimasa S, Yasui M, et al. Excellent outcome of allogeneic hematopoietic SCT with reduced-intensity conditioning for the treatment of chronic active EBV infection. Bone Marrow Transplant. 2011. 46:77–83.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download