Abstract
Extrinsic compression of the left main coronary artery (LMCA) secondary to pulmonary artery dilatation is a rare syndrome. Most cases of pulmonary artery hypertension but no atherosclerotic risk factors rarely undergo coronary angiography, and hence, diagnoses are seldom made and proper management is often delayed in these patients. We describe a patient that presented with pulmonary hypertension, clinical angina, and extrinsic compression of the LMCA by the pulmonary artery, who was treated successfully by percutaneous coronary intervention. Follow-up coronary angiography showed patent stent in the LMCA in the proximity of the dilated main pulmonary artery. This case reminds us that coronary angiography and percutaneous coronary intervention should be considered in pulmonary hypertension patients presenting with angina or left ventricular dysfunction.
Extrinsic compression of the left main coronary artery (LMCA) secondary to pulmonary artery dilatation is a rare syndrome that has been associated with severe pulmonary hypertension, and sometimes causes angina, left ventricular ischemia, or sudden death (1-3). In this report, we describe a patient that presented with pulmonary hypertension, clinical angina, and extrinsic compression of the LMCA by the pulmonary artery, who was treated successfully by percutaneous coronary intervention.
A 53-yr-old woman was admitted because of aggravated dyspnea over the previous month on December 13, 2011. The patient had a history of pulmonary tuberculosis and four years before admission, was diagnosed with asthma and pulmonary hypertension, and subsequently was managed for asthma and aggravated right heart failure. However, despite treatment, she complained of markedly limited exercise tolerance (New York Heart Association functional class III), paroxysmal nocturnal dyspnea, orthopnea, and occasional angina-like chest pain.
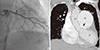
On admission, she was afebrile, with a blood pressure 100/70 mmHg, a pulse rate of 62 beats per minutes, and a respiratory rate of 20 breaths per minutes. Physical examination revealed a cachectic status (body weight 38 kg). In addition, neck veins were engorged, and soft abdominal distension, and bibasilar fine crackles on both lungs were appreciated. There were no signs of cyanosis or clubbing. Resting ECG showed a normal sinus rhythm, P pulmonale, and a right ventricular strain pattern with large biphasic QRS complexes in V2-V6. Chest radiography revealed an enlarged cardiac silhouette involving right chambers, and dilatation of the main pulmonary trunk, and right main pulmonary artery (Fig. 1). Computed tomography of the thorax confirmed these findings and demonstrated LMCA narrowing due to the compressive effect of the dilated pulmonary artery (Fig. 2). In addition, chronic thromboembolism was found in the right lower pulmonary artery. Transthoracic echocardiography (TTE) demonstrated a preserved left ventricular ejection fraction (LVEF) of 52%, an enlarged right atrium and right ventricle, a D-shaped left ventricle, and moderate pulmonary and tricuspid regurgitation, which corresponded to a right ventricular systolic pressure of 70 mmHg. TTE also shows a markedly dilated pulmonary artery trunk immediately adjacent to the take-off the LMCA (Fig. 3). A myocardial perfusion scan with Technetium-99m sestamibi revealed reversible perfusion defects in the apex, anterior wall, and septum. Coronary angiography of the right coronary artery showed minimal stenosis with grade 2 collaterals to the left circumflex artery and an arteriovenous fistula connecting with the pulmonary artery (Fig. 4A). Left coronary angiography demonstrated hypoplasia of the left coronary artery; only the diagonal branch was observed and not the left anterior descending (LAD) or left circumflex coronary artery (LCX). This study also revealed narrowing of the LMCA at its take-off from the aorta (Fig. 4B). The patient underwent ECG-gated, 128-slice multidetector computed tomography (MDCT) coronary angiography for further evaluation of stenosis of the LMCA, and extrinsic compression of the LMCA by the dilated pulmonary arterial trunk was documented (Fig. 5). Stenosis of the LMCA was treated by dilation and stenting with the intention of providing a metallic scaffold to resist the extrinsic compression. Briefly, a 0.014-inch guidewire (Run-through, Terumo, Japan) was passed through a 7Fr JL 4 guiding catheter with side hole and positioned in the ostial LMCA. Predilatation was performed using a 2.5 × 15 mm sized balloon (Ryujin plus, Terumo, Japan) in LMCA. After ballooning, the LAD and LCX were observed with LMCA dissection (Fig. 4C). The LMCA was then stented with a 32 mm long stent to a diameter of 2.75 mm (Promus Element, Boston Scientific, USA). Post-percutaneous coronary intervention showed no residual stenosis of the LMCA (Fig. 4D). Dual antiplatelet therapy, anticoagulation for chronic thromboembolism, and decongestive therapy for heart failure and optimal control of asthma were continued. At her 1-yr follow-up visit, coronary angiography and computed tomography with contrast showed a widely patent stent in the LMCA in the proximity of the dilated main pulmonary artery (Fig. 6). Even though there were no observable interval change of LVEF, chamber size and right ventricular systolic function by TTE, she reported improvement for in her functional capacity, chest pain and quality of life.
Left main coronary artery disease usually occurs secondary to atherosclerosis, although other conditions, such as, inflammatory pathologies and extrinsic compression, can lead to LMCA disease (4, 5). Among the secondary causes of LMCA compression, extrinsic compression by a dilated pulmonary artery is an increasingly recognized and potentially reversible cause of angina and left ventricular dysfunction in patients with pulmonary hypertension (6). This type of compression of the LMCA is primarily related to congenital heart disease or idiopathic pulmonary hypertension, but other causes of pulmonary hypertension can trigger this syndrome. Unlike atherosclerotic coronary artery disease, which is encountered more commonly in older males, extrinsic LMCA compression appears to affect younger patients and may have a higher incidence in women (7).
Extrinsic compression of the LMCA by a dilated pulmonary artery can lead to chest pain, left ventricular ischemia, or sudden death (8). The functional and prognostic significances of extrinsic left main coronary compression are not known, but malignant arrhythmia and left ventricular dysfunction caused by LMCA compression could contribute to a higher incidence of sudden death in patients with pulmonary hypertension (3, 9). In a previous case report, new symptoms in pulmonary hypertension were recognized to be secondary to extrinsic compression of the LMCA ostium by a dilated main pulmonary artery and were successfully relieved by the placement of a metallic stent in the affected segment of the LMCA (8). However, patients with severe pulmonary artery hypertension but no atherosclerotic risk factors rarely undergo coronary angiography, and hence, in these patients, diagnoses are seldom made and proper management is often delayed.
Currently, the gold standard for the diagnosis of LMCA compression is coronary angiography with IVUS. Other non-invasive techniques, such as, magnetic resonance imaging and multislice computed tomography may visualize the origins and courses of coronaries and allow the detection of significant coronary artery stenosis (10-12). Extrinsic compression of the coronary artery by an enlarged pulmonary artery trunk in patients with primary pulmonary hypertension is treatable, but if left untreated, can lead to left heart failure and death. Accordingly, some authors believe that coronary angiography should be performed on all primary pulmonary hypertension patients presenting with exertional angina or left ventricular dysfunction (13).
Dilatation of the pulmonary artery can lead to the displacement of the LMCA. In one study, invasive angiography demonstrated that the LMCA was inferiorly displaced (with a mean angle of 23° compared with 70° in the control group). In this study, it was suggested an LMCA origin in the right sinus of Valsalva indicates a higher risk of compression than more leftward origins (7). In another study on the risk factors predisposing LMCA compression in pulmonary artery hypertension, younger age, severe pulmonary arterial trunk dilation (PAT ≥ 40 mm, normal: ~25 to 30 mm), and a pulmonary trunk/aorta ratio of > 1.21 (normal:1.0) were found to be important parameters (14). Patients that should be considered for coronary angiography can be distinguished by measuring the diameter of the pulmonary trunk and its relation to aortic diameter echocardiographically.
Compression of the LMCA in patients with pulmonary hypertension is a treatable cause of angina and associated left ventricular ischemia. Numerous treatment options have been reported, but optimal treatment for this condition remains controversial. These options include percutaneous coronary intervention (PCI), coronary artery bypass grafting, surgical correction of congenital heart disease, pulmonary thromboendarterectomy, and heart-lung transplantation. In cases of primitive pulmonary hypertension, the most frequently adopted treatment is percutaneous stenting of the LMCA (9). Although bypass surgery remains the standard of care for patients with atherosclerotic LMCA disease, the 2009 ACC/AHA guidelines for PCI state that LMCA PCI is a class IIb recommendation that may be considered in patients with anatomic conditions associated with a low risk of procedural complications, or in patients with clinical conditions that predict an increased risk of adverse surgical outcomes (15). However, stenting of an unprotected LMCA has been associated with significant morbidity, and thus, in these high-risk patients, PCI would be best performed in a highly experienced center (16, 17). The type of stent, (drug-eluting stent (DES) or bare metal stent (BMS)), best suited for the treatment LMCA compression is debatable. The risk for restenosis is low due to the size of the LMCA and to the lack of atherosclerosis in those with LMCA compression. If the vessel is ≤ 3.5 mm in diameter, a DES may be preferable. However, although drug-eluting stents can prevent restenosis by inhibiting neointimal growth, long-term dual antiplatelet therapy is warranted to guard against catastrophic acute stent thrombosis in the left main coronary ostium (8, 9).
In conclusion, pulmonary hypertension and chest discomfort may be caused by extrinsic compression of LMCA, and thus, we recommend consideration of this syndrome in the differential diagnosis of angina or new left ventricular systolic dysfunction in patients with severe pulmonary artery hypertension. Extrinsic compression of LMCA can be diagnosed by CT or MRI and confirmed by invasive angiography, and at experienced centers can be treated safely by PCI and achieve good angiographic results. Further studies are required to ascertain the impact of PCI on the prognoses of these patients.
Figures and Tables
Fig. 1
Chest X-ray. Cardiomegaly involving right chambers, enlarged pulmonary trunk (arrow), and right main pulmonary artery (head arrow).
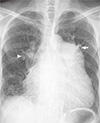
Fig. 2
Chest CT scan with contrast. (A) Markedly dilated pulmonary trunk (68mm) and pulmonary arteries. (B) Dilated main pulmonary artery trunk pressing against left main coronary artery. Arrows point to compression. AO, aorta; PA, main pulmonary artery trunk; LV, Left ventricle.
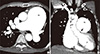
Fig. 3
Transthoracic echocardiogram shows evidence of a dilated main pulmonary artery trunk pressing against left main coronary artery.
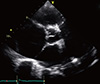
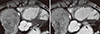
Fig. 4
Coronary angiography. (A) Right coronary angiography demonstrated minimal stenosis with grade 2 collaterals to the left circumflex artery and an arteriovenous fistula connecting with pulmonary artery. (B) Left coronary angiography demonstrated hypoplasia of left coronary artery and only diagonal branch was observed without left anterior descending (LAD) and left circumflex coronary artery (LCX). The study also reveals narrowing of the LMCA at its take-off from the aorta (arrow). (C) After balloon angioplasty, LAD and LCX was observed with LMCA dissection (arrow). (D) After stenting of the left main stenosis, excellent results with wide lumen of the left main coronary artery.

References
1. Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK, et al. Primary pulmonary hypertension: a national prospective study. Ann Intern Med. 1987; 107:216–223.
2. Vizza CD, Lynch JP, Ochoa LL, Richardson G, Trulock EP. Right and left ventricular dysfunction in patients with severe pulmonary disease. Chest. 1998; 113:576–583.
3. D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med. 1991; 115:343–349.
4. Kumar GV, Agarwal NB, Javali S, Patwardhan AM. Takayasu's arteritis with ostial and left main coronary artery stenosis. Tex Heart Inst J. 2007; 34:470–474.
5. Rubín JM, Arias JC, Lambert JL. Unusual case of coronary stenosis caused by an external compression. Int J Cardiol. 1997; 62:167–169.
6. Lee MS, Oyama J, Bhatia R, Kim YH, Park SJ. Left main coronary artery compression from pulmonary artery enlargement due to pulmonary hypertension: a contemporary review and argument for percutaneous revascularization. Catheter Cardiovasc Interv. 2010; 76:543–550.
7. Kajita LJ, Martinez EE, Ambrose JA, Lemos PA, Esteves A, Nogueira da Gama M, Jatene AD, Ramires JA. Extrinsic compression of the left main coronary artery by a dilated pulmonary artery: clinical, angiographic, and hemodynamic determinants. Catheter Cardiovasc Interv. 2001; 52:49–54.
8. Sivakumar K, Rajan M, Francis G, Murali K, Bashi V. Extrinsic compression of the left coronary ostium by the pulmonary trunk: management in a case of Eisenmenger syndrome. Tex Heart Inst J. 2010; 37:95–98.
9. Lindsey JB, Brilakis ES, Banerjee S. Acute coronary syndrome due to extrinsic compression of the left main coronary artery in a patient with severe pulmonary hypertension: successful treatment with percutaneous coronary intervention. Cardiovasc Revasc Med. 2008; 9:47–51.
10. Kawut SM, Silvestry FE, Ferrari VA, DeNofrio D, Axel L, Loh E, Palevsky HI. Extrinsic compression of the left main coronary artery by the pulmonary artery in patients with long-standing pulmonary hypertension. Am J Cardiol. 1999; 83:984–986.
11. Schuijf JD, Jukema JW, van der Wall EE, Bax JJ. Multi-slice computed tomography in the evaluation of patients with acute chest pain. Acute Card Care. 2007; 9:214–221.
12. De Jesus Perez VA, Haddad F, Vagelos RH, Fearon W, Feinstein J, Zamanian RT. Angina associated with left main coronary artery compression in pulmonary hypertension. J Heart Lung Transplant. 2009; 28:527–530.
13. Rich S, McLaughlin VV, O'Neill W. Stenting to reverse left ventricular ischemia due to left main coronary artery compression in primary pulmonary hypertension. Chest. 2001; 120:1412–1415.
14. Di Salvo G, Eyskens B, Claus P, D'hooge J, Bijnens B, Suys B, De Wolf D, Gewillig M, Sutherland GR, Mertens L. Late post-repair ventricular function in patients with origin of the left main coronary artery from the pulmonary trunk. Am J Cardiol. 2004; 93:506–508.
15. Kushner FG, Hand M, Smith SC Jr, King SB 3rd, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009; 54:2205–2241.
16. Park SJ, Park SW, Hong MK, Cheong SS, Lee CW, Kim JJ, Hong MK, Mintz GS, Leon MB. Stenting of unprotected left main coronary artery stenoses: immediate and late outcomes. J Am Coll Cardiol. 1998; 31:37–42.
17. Silvestri M, Barragan P, Sainsous J, Bayet G, Simeoni JB, Roquebert PO, Macaluso G, Bouvier JL, Comet B. Unprotected left main coronary artery stenting: immediate and medium-term outcomes of 140 elective procedures. J Am Coll Cardiol. 2000; 35:1543–1550.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download