Abstract
Crossed aphasia (CA) refers to language impairment secondary to right hemisphere lesion. Imaging analysis on the lesion location of CA has not yet been reported in the literature. This study was proposed to analyze the most prevalent lesion site related to CA. Brain MRI of 7 stroke patients satisfying the criteria for CA were used to define Region of interest (ROIs) before overlaying the images to visualize the most overlapped area. Talairach coordinates for the most overlapped areas were converted to corresponding anatomical regions. Anatomical lesions where more than 3 patients' images were overlapped were considered significant. The overlayed ROIs of 7 patients revealed the lentiform nucleus as the most frequently involved area, overlapping in 6 patients. Our study first demonstrates the areas involved in CA by lesion mapping using brain MRI, and lentiform nucleus is the responsible neural substrate for crossed aphasia.
Aphasia is one of main manifestations of stroke, which is most common cause of long term disability. Croquelois and Bogousslavsky (1) reported in their study of 1,500 consecutive cases of post-stroke aphasia, 1,387 (90%) had left hemisphere lesion while 79 (5%) had lesion in the right hemisphere and the rest 75 (5%) bilateral lesion.
Crossed aphasia (CA) was first defined as a language disturbance after right hemispheric stroke in dextrals (2). Majority of aphasia in right handed individuals are caused by left hemisphere stroke, and crossed aphasia following a right hemispheric lesion is rarely observed. The prevalence of CA in right handed patients is reported to be between 0.38 and 3% of all aphasic syndromes. The diagnostic criteria for CA are: 1) aphasia; 2) lesion in the right unilateral hemisphere; 3) strong preference for right hand use without familial history of left handedness; 4) structural integrity of the left hemisphere; and 5) absence of brain damage in childhood (3). Many cases have been reported over the last decade but precise mechanisms underlying language disorders of crossed aphasia are not yet completely understood (4). Proposed explanations for crossed aphasia include 1) a previously silent or unrecognized lesion in the left hemisphere that is somehow rendered symptomatic by a new lesion in the right hemisphere, 2) ipsilateral control of the dominant hand, 3) bilateral representation of linguistic functions; and 4) an arrested developmental stage in the lateralization of language function (2). The patterns of lesion distribution and recovery are reported to resemble those of uncrossed aphasia. Despite both oral and written modes of language comprehension being rarely induced by right hemispheric stroke aphasia, CA does not account for all right hemisphere lesion causing language impairments (5). Theories about the pathogenesis, clinical manifestations and lesion sites of crossed aphasia are still controversial despite many case reports in the literature. However, demonstration of the anatomical lesion accounting for CA has never been explicitly demonstrated, and no study on brain mapping of the frequent involved site related to CA has been performed to our knowledge up to now.
Therefore, we performed mapping of brain MRI images of seven aphasic stroke patients with ischemic lesion limited to the right hemisphere, to localize the region responsible for the CA.
Aphasic patients with right hemisphere lesions from 2005 to 2011 were retrospectively reviewed through medical records. The inclusion criteria other than the definitions of CA (See introduction for diagnostic criteria for CA) are as following: 1) first ever stroke; 2) no history of previous aphasia of any kind prior to stroke; 3) presence of initial brain MRI images within 3 days of stroke onset (only the initial MRI was analyzed); 4) record of initial speech evaluation within 3 weeks of stroke onset; without hearing loss or difficulties; 5) and those without tracheostomy. Brain MRI images and speech evaluation data of 9 patients with aphasia and right hemisphere lesion were gathered and their findings were recorded. Out of the 9 patients, one patient was excluded because the speech evaluation revealed severe cognitive impairment affecting conversation, rather than true aphasia, and another patient was excluded because speech evaluation was not carried out completely due to poor cooperation. The demographic data included subjects' years of education, onset and the number of days from stroke onset to initial speech evaluation. Korean version of Western Aphasia Battery (KWAB) (6), an instrument for assessing the language function of adults, was used to discern the presence, degree, and type of aphasia. The categories of KWAB included spontaneous speech, comprehension, repetition, naming and aphasia quotient (AQ). The AQ is the summary score that indicates overall severity of language impairment. The brain MRI images, especially the T1 and FLAIR views, were thoroughly reviewed with reference to the official readings by expert neuroradiologist to make sure there are no bilateral lesions.
MRI was conducted using a 1.5-T unit (Intera, Philips Medical Systems, Best, The Netherlands) with a sensitivity-encoding (SENSE) head coil, and head/neck synergy coil. Fluid attenuated inversion recovery images (acquired voxel size = 0.5 × 0.5 × 1 µL, transverse orientation) was used for visualizing the lesion. A total of 68 axial images were collected for each subject, encompassing the whole-brain. DICOM files collected from scanning were acquired and spatially normalized into reconstructed images of isotropic voxel size of 2 × 2 × 2 µL using SPM8 implemented in Matlab (Version 7.8.0. The Mathworks Inc, Natick, MA, USA) (7).
MRIcro was used to manually define outline of the hyperintense lesions in the spatially normalized FLAIR images with reference to the co-registered T1-weighted images for additional guidance by a physician blind to patients' speech impairment (8) using Bamboo™ (Wacom) program to enhance precision. Out of the total 68 axial slices, 34th slice was set as the median level and 3 additional levels both below and above the 34th slice were selected, with total of 7 levels, for analysis. The 7 levels were separated by 5 slices, and region of interest (ROI) were drawn at the slices of 19, 24, 29, 34, 39, 44, and 49. Seven slices of the patients were overlayed at each level to overlap the ROIs and visualize the overlapped areas. The overlapped areas were displayed by use of a color coding to depict the number of overlapped ROIs (red = 7, yellow = 6, lime = 5, green = 4, blue = 3, navy = 2, purple = 1) at each level. After completing above process for all of the selected slices, talairach space coordinate of the exact anatomical site of the most overlapped area was marked at the center of each most overlapped region using the MRIcro software. The talairach space coordinates gained were then inputted to the Talairach-Client program to convert the coordinates into the anatomical names at the marked coordinate as well as anatomical structures within 5 mm or nearest gray matter of the centre of the ROI. Anatomical lesions at which more than 3 patients' images were overlapped were considered significant, and the names of anatomical regions were listed in the order of frequency of appearance.
The patients' (4 males) age at the time of stroke ranged from 51 to 74 yr, with mean of 68 yr. The duration from onset of the stroke to the initial speech evaluation was from 6 to 21 days, with mean of 12.7 days. The total years of education in their lifetime ranged from 0 to 9 yr with mean of 4.3 yr. The patients were all right handed (judged by the Edinburgh inventory) (9) with no family history of sinistrality (Table 1). The aphasia types were transcortical motor (n = 3), global (n = 2), anomic (n = 1), and motor (n = 1). KWAB results are summarized in Table 2. Spontaneous speech assessment results ranged from 13% to 81%, comprehension 3% to 89%, repetition 1% to 99%, naming 11% to 88% and AQ from 14% to 78%, showing wide range in every categories of the speech evaluation (Table 2). The sites of lesion in the right hemisphere were frontal, temporal, parietal lobes, basal ganglia and thalamus. There was no specific characteristic pattern or correlation between the lesions sites and aphasia types. As for the site of lesions per patient, parietal lobe involvement was always accompanied by involvement in the temporal lobe (in 3 of the patients), whose aphasia types all differed (global, motor and transcortical motor). Basal ganglia was involved in 2 patients, while thalamus and frontal lobe were involved in 3 patients, also showing no specific relationship with the types of aphasia.
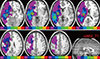
Five patients overlapped with talairach space coordinates at the center of the ROI of -32×-22×-14 in the first slice, 6 patients overlapped with talairach space coordinate of -30×-12×-4 in the second slice, 5 patients overlapped with coordinates of -30×-14×6 in the third slice, in the fourth slice, 5 patients overlapped with coordinates of -28×-18×16, and in the fifth slice 4 patients overlapped with coordinate of -34×-8×26. In the sixth and seventh slice, less than 3 patients' images overlapped and were therefore not anatomically analyzed. Fig. 1 shows the axial view of each level with open circle indicating the region with the highest number of ROIs (more than 4 overlaps). The last two slices (44 and 49) has no indicating open circle since the ROI overlap is less than 3. The sagittal slice in the right below shows the level of the 7 slices at which the analysis of ROI was performed. The overlayed ROIs of 7 patients revealed the commonly involved areas as following: lentiform nucleus was overlapped in 6 patients, limbic lobe, the parahippocampal gyrus and claustrum were overlapped in 5 patients and, frontal lobe and precentral gyrus were overlapped in 4 patients. The lentiform nucleus was observed as the most frequently involved areas in CA patients, and slice 24 was the level with most ROI overlaps.
The lentiform nucleus was found to be the most frequently involved neural substrate responsible for CA, overlapping in 6 out of 7 patients. So far, mapping of the lesion to find out the common site resulting in CA in dextral has not yet been undertaken. Although lentiform nucleus and the putamen have been suggested to be the lesions underlying CA in several case reviews, specific imaging analysis was not carried out. This is the first study to analyze MRI images to localize the frequently involved area by visualizing the overlapped lesion areas in the CA patients.
In reviewing clinical features of acute infarctions limited to the lentiform nucleus, it was revealed that acute lenticular infarction induces mainly hemiparesis, and associated sensory deficits, aphasia, and hemineglect underline the function of lentiform nucleus in connection with the prefrontal, temporal, and parietal cortices (10). In a longitudinal PET study, aphasic patients showing higher activity in right thalamus and lentiform nucleus significantly recovered better than those who did not at one year post-stroke, suggesting that right thalamus and lentiform nuclei contribute to aphasia and its recovery (11). In a report of one CA patient with involvement of the lentiform nucleus, the caudate nucleus and the internal capsule, cortical regions known to be of some relevance for language - supra-marginal, angular gyrus and the superior temporal gyrus, were spared. Furthermore, relating to two other cases, intra-hemispheric deep locations involving parts of basal ganglia, putamen and caudate were described as the common areas responsible for aphasia. They made an observation that deep structures, solely or not, are mostly involved in CA, unlike aphasia caused by left hemisphere lesion, and there may be a relationship between the involvement of right basal ganglia and occurrence of CA (12). Despite frequently mentioned right basal ganglia, putamen and lentiform nucleus in the case reviews of CA patients, the pathophysiology explaining their involvement had not been fully given, or were left inconclusive yet, when it came to correlating the lesion site and aphasia. Pure ischemic stroke limited to putamen has been rarely reported. Most putaminal lesion resulted from head trauma, and not limited to the putamen, usually involving adjacent structures (10). In a study of patients with lenticular infarcts which attempted to classify the types of clinical syndromes, 5 out of 16 cases of putaminal infarction showed language impairment, but they were all left side infarcts. The speech disorders seen among these patients were transcortical type of aphasia, characterized by hypophonia, reduced output, verbal paraphasias, and preserved repetition (13). Likewise, as seen in our results, in spite of lentiform nucleus being the most frequently involved site of lesion, none of the cases were limited only to the lentiform nucleus.
The most common cause of CA has been reported to be secondary to vascular lesions (2). The importance of careful control for etiology when suspecting CA has been argued. In a case report of CA whose initial brain CT showed hemorrhagic lesion only in the right frontal area while EEG revealed left occipitotemporal dysfunction that accounted for aphasic manifestations. The authors did not include traumatic cases, suggesting much higher incidence of CA in traumatic patients owing to undetected countercoup lesion in the left hemisphere (15). However, all of our 7 patients had ischemic lesions, therefore avoiding the possibility of accompanying left hemispheric dysfunction that could potentially induce aphasic symptoms. PET study of CA suggested that diaschisis might be responsible for the occurrence of CA, for example the presence of functional depression of language areas in the left hemisphere together with structurally abnormal lesions in the right hemisphere (15).
The theory of unilateral right hemisphere involvement in CA was further supported by SPECT study of a patient who developed non-fluent aphasia after resection of right parietal arteriovenous malformation. The SPECT study showed reduced perfusion only in the right hemisphere as well as language activation only in the right frontal lobe after naming task (16). Furthermore, abnormal dominance for some language functions in the right hemisphere underlying the syndrome of CA was demonstrated in 3 CA cases (2). In our study, MRI of the 7 subjects taken in the very acute stage showed right hemisphere lesion only, supporting the right hemisphere dominance as the basis of CA, rather than diaschisis (2). Role of the right hemisphere in speech process is not as obvious as the left hemisphere, and involvement of left basal ganglia in speech is also not yet clearly identified. Disinhibited speech behaviors in, both deep and superficial lesions, display less precise specificity than the cortical structures. In analyzing published literatures on speech disorders of right hemispheric stroke in terms of locations, speech disorder types and possible mechanisms, poor understanding of mechanisms for the subcortical CA was pointed out and also that there is no single theory providing a complete explanation of the speech dysfunction in CA (17).
The most common type of crossed aphasia was the non-fluent aphasia (72%), and only 27% of cases were fluent aphasia (18). These findings parallel with the results of present study, in which initial speech evaluation of all 7 patients showed non-fluent aphasia. The characteristics of language functions in the right hemisphere in CA patients are widely represented and that lesion anywhere in the right hemisphere produced non-fluent aphasia. In Basso et al.'s study, the correlation between the aphasia type and the locus of lesion differed in patients with CA from the patients with aphasia secondary to left hemisphere lesion (19). However, on our reviewing of the published literature on CA from 1975 to 2004, it was also suggested that CA does not include more patients with non-fluent than fluent aphasia, and that the nonverbal neuropsychological impairments such as visuospatial neglect and apraxia are associated with CA (20). A case of crossed Wernicke's aphasia was also reported (21), and non-fluent CA following the right corpus callosum infarction was recently reported (3). All of our 7 patients had non-fluent aphasia and 2 out of the 3 patients with focal right basal ganglia infarction showed transcortical motor aphasia while one showed anomic aphasia.
Due to rarity of CA in dextral and its strict criteria, our retrospective study was performed with some limitations. The number of patients was small to ascertain that lentiform nucleus is the most commonly involved site in the development of CA, requiring more cases to support our findings. Also, only the initial speech evaluation was recorded, thus follow up evaluation to find out the prognosis of aphasia, or changes in type of aphasia over time was not performed. Additional examinations other than brain imaging, such as EEG and SPECT were not performed to further evaluate brain function in the left hemisphere, thus we have to take into consideration the undetected underlying left hemisphere lesion contributing to aphasia. Moreover, role of the lentiform nucleus and its linguistic function is yet lacking in the literature, and thus to be further elucidated in the future for better understanding of CA.
This study is the first to demonstrate the areas involved in CA by lesion mapping using brain MRI. The lentiform nucleus was the most frequently involved, overlapping in 6 out of 7 patients.
In conclusion, lentiform nucleus seems to be the neural substrate responsible for CA. More studies with larger number of cases and use of functional neuroimaging, together with more extensive correlation with the aphasia patterns would further enhance our knowledge in the future.
Figures and Tables
Fig. 1
FLAIR MR images of axial brain slices of 7 patients showing distribution of all patients lesion area on a brain template. Color-coding reflects number of patients with local lesion overlap (red = 7, yellow = 6, lime = 5, green = 4, blue = 3, navy = 2, purple = 1). Red empty circles indicate the most overlapped anatomical regions. The sagittal sections (lower, right) indicate each level of analysis.
Notes
References
1. Croquelois A, Bogousslavsky J. Stroke aphasia: 1,500 consecutive cases. Cerebrovasc Dis. 2011; 31:392–399.
2. Bakar M, Kirshner HS, Wertz RT. Crossed aphasia: functional brain imaging with PET or SPECT. Arch Neurol. 1996; 53:1026–1032.
3. Ishizaki M, Ueyama H, Nishida Y, Imamura S, Hirano T, Uchino M. Crossed aphasia following an infarction in the right corpus callosum. Clin Neurol Neurosurg. 2012; 114:161–165.
4. Castro-Caldas A, Confraria A. Age and type of crossed aphasia in dextrals due to stroke. Brain Lang. 1984; 23:126–133.
5. Dewarrat GM, Annoni JM, Fornari E, Carota A, Bogousslavsky J, Maeder P. Acute aphasia after right hemisphere stroke. J Neurol. 2009; 256:1461–1467.
6. Kim H, Na DL. Normative data on the Korean version of the Western Aphasia Battery. J Clin Exp Neuropsychol. 2004; 26:1011–1020.
7. Marchina S, Zhu LL, Norton A, Zipse L, Wan CY, Schlaug G. Impairment of speech production predicted by lesion load of the left arcuate fasciculus. Stroke. 2011; 42:2251–2256.
8. Van Oers CA, Vink M, van Zandvoort MJ, van der Worp HB, de Haan EH, Kappelle LJ, Ramsey NF, Dijkhuizen RM. Contribution of the left and right inferior frontal gyrus in recovery from aphasia: a functional MRI study in stroke patients with preserved hemodynamic responsiveness. Neuroimage. 2010; 49:885–893.
9. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971; 9:97–113.
10. Russmann H, Vingerhoets F, Ghika J, Maeder P, Bogousslavsky J. Acute infarction limited to the lenticular nucleus: clinical, etiologic, and topographic features. Arch Neurol. 2003; 60:351–355.
11. De Boissezon X, Marie N, Castel-Lacanal E, Marque P, Bezy C, Gros H, Lotterie JA, Cardebat D, Puel M, Demonet JF. Good recovery from aphasia is also supported by right basal ganglia: a longitudinal controlled PET study: EJPRM-ESPRM 2008 award winner. Eur J Phys Rehabil Med. 2009; 45:547–558.
12. Habib M, Joanette Y, Ali-Cherif A, Poncet M. Crossed aphasia in dextrals: a case report with special reference to site of lesion. Neuropsychologia. 1983; 21:413–418.
13. Giroud M, Lemesle M, Madinier G, Billiar T, Dumas R. Unilateral lenticular infarcts: radiological and clinical syndromes, aetiology, and prognosis. J Neurol Neurosurg Psychiatry. 1997; 63:611–615.
14. Castro-Caldas A, Confraria A, Paiva T, Trindade A. Contrecoup injury in the misdiagnosis of crossed aphasia. J Clin Exp Neuropsychol. 1986; 8:697–701.
15. Cappa SF, Perani D, Bressi S, Paulesu E, Franceschi M, Fazio F. Crossed aphasia: a PET follow up study of two cases. J Neurol Neurosurg Psychiatry. 1993; 56:665–671.
16. Gomez-Tortosa E, Martin EM, Sychra JJ, Dujovny M. Language-activated single-photon emission tomography imaging in the evaluation of language lateralization: evidence from a case of crossed aphasia: case report. Neurosurgery. 1994; 35:515–519.
17. Dyukova GM, Glozman ZM, Titova EY, Kriushev ES, Gamaleya AA. Speech disorders in right-hemisphere stroke. Neurosci Behav Physiol. 2010; 40:593–602.
18. Carr MS, Jacobson T, Boller F. Crossed aphasia: analysis of four cases. Brain Lang. 1981; 14:190–202.
19. Basso A, Capitani E, Laiacona M, Zanobio ME. Crossed aphasia: one or more syndromes? Cortex. 1985; 21:25–45.
20. Mariën P, Paghera B, De Deyn PP, Vignolo LA. Adult crossed aphasia in dextrals revisited. Cortex. 2004; 40:41–74.
21. Sheehy LM, Haines ME. Crossed Wernicke's aphasia: a case report. Brain Lang. 2004; 89:203–206.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download