Abstract
Reported herein is an adult case of Fisher syndrome (FS) that occurred as a complication during the course of community-acquired pneumonia caused by Mycoplasma pneumoniae. A 38-yr-old man who had been treated with antibiotics for serologically proven M. pneumoniae pneumonia presented with a sudden onset of diplopia, ataxic gait, and areflexia. A thorough evaluation including brain imaging, cerebrospinal fluid examination, a nerve conduction study, and detection of serum anti-ganglioside GQ1b antibody titers led to the diagnosis of FS. Antibiotic treatment of the underlying M. pneumoniae pneumonia was maintained without additional immunomodulatory agents. A complete and spontaneous resolution of neurologic abnormalities was observed within 1 month, accompanied by resolution of lung lesions.
Neurologic impediments transpire in 0.1% of patients infected with Mycoplasma pneumoniae (1). Although direct intracerebral infection can occur in patients with M. pneumoniae disease, neurotoxic or autoimmune reactions are the most common neurologic complications (1, 2). These complications include Guillain-Barre syndrome (GBS), a disorder in which the immune system attacks the nerves, as well as its variants (3, 4).
Fisher syndrome (FS) is a variant of GBS that is characterized by the acute onset of ophthalmoplegia, ataxia, and areflexia (5). Anti-ganglioside GQ1b antibodies are also found in the serum of afflicted individuals (6). Unlike GBS itself, FS has rarely been linked to M. pneumoniae infection (7). We report herein an adult case of anti-GQ1b antibody-positive FS associated with community-acquired M. pneumoniae pneumonia. To our knowledge, such case has rarely been described since the discovery of the anti-GQ1b antibody in 1993 (6).
A 38-yr-old man presented to our emergency room complaining of a 2-week history of productive cough followed by fever on January 10, 2012. He was previously treated for respiratory tract infection at a private clinic, but there was no improvement in his symptoms. His past medical history was unremarkable. He appeared acutely ill. His blood pressure was 90/60 mmHg, temperature 101℉, respiratory rate 22/min, and pulse rate 108 beats/min.
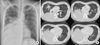
On examination, crackles were present in the right lower lung. A chest radiograph showed consolidation in the entire right lower lung field (Fig. 1A). Computed tomography of the chest demonstrated lobar consolidation of the right lower lobe, accompanied by some patchy consolidation in the right middle and left upper lobe (Fig. 1B). No evidence of pleural effusion was observed. Laboratory tests showed normal complete blood cell counts, an erythrocyte sedimentation rate of 65 mm/hr (0-10 mm/hr), and C-reactive protein levels of 19.2 mg/dL (< 0.5 mg/dL). Oxygen saturation was 94% in room air. A urine antigen analysis for Streptococcus pneumoniae and Legionella pneumophila tested negative for both organisms. Procalcitonin level was 0.35 ng/mL (0-0.05 ng/mL). Testing for IgM and IgG antibodies against M. pneumoniae showed elevated titers for both (Table 1). Testing for cold agglutinin disease also revealed positive findings (1:32). A diagnosis of pneumonia subsequent to M. pneumoniae infection was made, and azithromycin was intravenously administered.
On the second hospital day, the patient began to complain of a bilateral headache and diplopia. A neurologic examination revealed the pupils to be equal and responding to light. However, ocular movements were impaired. Prominent limitation of supraduction and mild limitation of abduction were discerned. Head turning and nodding did not influence eye movement. Nystagmus was absent. The visual acuity was not decreased bilaterally, and no signs of meningeal irritation were present. Deep reflexes were decreased. A wide-based ataxic gait was observed, although motor power in the arms and legs was good. Sensation was within normal limits, but a Romberg test to detect poor balance was positive. Brain magnetic resonance imaging (MRI) revealed no abnormalities.
A lumbar puncture was performed and revealed a slightly increased white blood cell count and elevated protein levels (Table 1). Cerebrospinal fluid (CSF) glucose and adenosine deaminase levels were normal. Cultures for bacteria, virus, and fungi were negative, and CSF polymerase chain reaction tests for Mycobacterium tuberculosis, herpes simplex virus, cytomegalovirus, Epstein-Barr virus and M. pneumoniae were all negative. Nerve conduction tests showed decreased sensory nerve action potentials of the right sural nerve. Motor nerve conduction velocity of the right common peroneal nerve was decreased. The blink reflex was normal. Additionally, serological tests for IgM and IgG antibodies against anti-GT1a and GM1 antibody were all negative, but the test for anti-GQ1b IgG antibody was positive.
A diagnosis of FS with antecedent M. pneumoniae pneumonia was made, and treatment of the underlying pneumonia was maintained. Three days later, the patient's body temperature had normalized, and the productive cough was somewhat suppressed. However, headache and diplopia continued. Five days after azithromycin administration, the headache and ataxia began to subside, but diplopia had not resolved. One week after admission, the respiratory symptoms were absent, and diplopia began to improve slowly. Limitation of supraduction resolved earlier than limitation of abduction. Improvement thereafter was gradual, and the patient was discharged 10 days after admission. The ataxic gait and areflexia were completely recovered, but remnant mild diplopia remained at the time of discharge. Follow-up testing for antibodies against M. pneumoniae showed still elevated IgM (8.5 index) and more elevated titers for IgG (> 100 AU/mL) a week after discharge. The patient returned 1 month later for additional follow-up. His ocular examination revealed complete resolution of ocular motility.
M. pneumoniae infections can involve many systems of the body in addition to the respiratory tract, including the nervous system (2). Neurologic manifestations are the most common extrapulmonary complications that are related to significant morbidity (8). Although the exact mechanisms underlying neurological disease following M. pneumoniae infection remain unknown, a number of possibilities have been set forth (2, 8, 9), such as immune complex-mediated injury (9). The cell membrane of M. pneumoniae contains lipoproteins that are potent inducers of inflammatory cytokines (10). Furthermore, the cytoplasm of M. pneumoniae contains potent immunogenic substances (e.g., glycolipids and glycoproteins) that can elicit autoimmunity through molecular mimicry of various human cellular components such as those of brain tissue (11).
GBS is a representative neurologic complication that may be best explained by immune complex-mediated injury associated with M. pneumoniae infection (12-14). In addition to M. pneumoniae, Campylobacter jejuni, Haemophilus influenzae, cytomegalovirus, and Epstein-Barr virus are known to be causative factors for GBS (15). Molecular mimicry is the most likely mechanism by which all of these infectious agents trigger an immunological reaction (15).
FS is regarded as a clinical variant of GBS and is defined by a characteristic triad of acute ophthalmoplegia, ataxia, and areflexia, based on Fisher's original article (5). Like GBS, FS has been reported to follow infections, the majority of which affect the respiratory tract (3, 15, 16). To date, a large prospective case-controlled study has shown evidence for C. jejuni and H. influenzae antecedent infection in FS patients (17). However, other GBS-associated pathogens such as M. pneumoniae are also possible candidates for the precipitation of FS (14, 18). However, a relatively infrequent incidence of FS compared with GBS might weaken the association between FS and M. pneumoniae infection (18).
In 1994, Merkx et al. (7) reported a typical adult case of FS as a neurologic manifestation after M. pneumoniae infection. However, the case was diagnosed on the basis of FS-compatible clinical symptoms alone, because the presence of the anti-GQ1b antibody had not yet been linked with FS. The anti-GQ1b antibody is present in the serum of about 80%-95% of the FS patients and is now widely used as a diagnostic marker of FS (16).
GQ1b is a ganglioside subtype that is present in cranial nerves and in the pre-synaptic terminals of neuromuscular junctions (18). Infections by bacteria bearing the GQ1b epitope may induce the production of anti-GQ1b antibodies in the host via molecular mimicry. For example, Yuki et al. (15) demonstrated that monoclonal antibody clones reacted with GQ1b gangliosides and lipopolysaccharide epitopes located in the outer membrane of C. jejuni. The anti-GQ1b antibody elicited in the host in response to the foreign epitope then attacks the GQ1b ganglioside on the surface of cranial nerves involved in ocular movements. The net result is inflammation, subsequent disruption of cranial nerve functions (6, 16), and opthalmoplegia in FS patients. While M. pneumoniae is now considered to be a possible candidate for molecular mimicry in FS (15), it remains to be verified whether M. pneumoniae does in fact express the GQ1b epitope and whether autoantibodies are produced in the host as a result of antigen alteration during infection.
Besides the adult FS case documented by Merkx et al. (7), a few anti-GQ1b antibody-positive FS cases in children after M. pneumoniae infection have been reported (19). The case described herein represents the rare description of an adult case of FS associated with M. pneumoniae pneumonia in which antibodies against both M. pneumoniae and GQ1b ganglioside were simultaneously identified. This suggests that M. pneumoniae has etiological significance for the development of FS.
The effect of immunomodulatory treatment such as immunoglobulin or plasma exchange is a controversial issue in FS (18, 20). Interventions other than the administration of azithromycin for M. pneumoniae infection were not applied in the current case, and the patient showed complete recovery of all neurologic manifestations within 1 month.
In conclusion, an adult case of FS showed a spontaneous and complete recovery after treatment of the underlying M. pneumoniae pneumonia. Therefore, the possibility of an association between FS and M. pneumoniae infection should be considered in adult pneumonia patients complicated with neurologic manifestations, such as ophthalmoplegia and ataxia.
Figures and Tables
References
1. Koskiniemi M. CNS manifestations associated with Mycoplasma pneumoniae infections: summary of cases at the University of Helsinki and review. Clin Infect Dis. 1993. 17:S52–S57.
2. Cassell GH, Cole BC. Mycoplasmas as agents of human disease. N Engl J Med. 198. 304:80–89.
3. Lo YL. Clinical and immunological spectrum of the Miller Fisher syndrome. Muscle Nerve. 2007. 36:615–627.
4. Loewenbruck KF, Putz V, Schafer J, Reichmann H, Storch A. Parainfectious polyneuropathy and miller-fisher-syndrome in combination with anemia in Mycoplasma pneumoniae infection. Fortschr Neurol Psychiatr. 2008. 76:361–365.
5. Fisher M. An unusual variant of acute idiopathic polyneuritis (syndrome of ophthalmoplegia, ataxia and areflexia). N Engl J Med. 1956. 255:57–65.
6. Chiba A, Kusunoki S, Obata H, Machinami R, Kanazawa I. Serum anti-GQ1b IgG antibody is associated with ophthalmoplegia in Miller Fisher syndrome and Guillain-Barre syndrome: clinical and immunohistochemical studies. Neurology. 1993. 43:1911–1917.
7. Merkx H, De Keyser J, Ebinger G. Miller Fisher syndrome associated with Mycoplasma pneumoniae infection: report of a case. Clin Neurol Neurosurg. 1994. 96:96–99.
8. Tsiodras S, Kelesidis I, Kelesidis T, Stamboulis E, Giamarellou H. Central nervous system manifestations of Mycoplasma pneumoniae infections. J Infect. 2005. 51:343–354.
9. Narita M. Pathogenesis of neurologic manifestations of Mycoplasma pneumoniae infection. Pediatr Neurol. 2009. 41:159–166.
10. Yang J, Hooper WC, Phillips DJ, Talkington DF. Cytokines in Mycoplasma pneumoniae infections. Cytokine Growth Factor Rev. 2004. 15:157–168.
11. Biberfeld G. Antibodies to brain and other tissues in cases of Mycoplasma pneumoniae infection. Clin Exp Immunol. 1971. 8:319–333.
12. Jacobs BC, Rothbarth PH, van der Meche FG, Herbrink P, Schmitz PI, de Klerk MA, van Doorn PA. The spectrum of antecedent infections in Guillain-Barre syndrome: a case-control study. Neurology. 1998. 51:1110–1115.
13. Goldschmidt B, Menonna J, Fortunato J, Dowling P, Cook S. Mycoplasma antibody in Guillain-Barre syndrome and other neurological disorders. Ann Neurol. 1980. 7:108–112.
14. Kitazawa K, Tagawa Y, Honda A, Yuki N. Guillain-Barre syndrome associated with IgG anti-GM1b antibody subsequent to Mycoplasma pneumoniae infection. J Neurol Sci. 1998. 156:99–101.
15. Yuki N. Infectious origins of, and molecular mimicry in, Guillain-Barre and Fisher syndromes. Lancet Infect Dis. 2001. 1:29–37.
16. Mori M, Kuwabara S, Yuki N. Fisher syndrome: clinical features, immunopathogenesis and management. Expert Rev Neurother. 2012. 12:39–51.
17. Koga M, Gilbert M, Li J, Koike S, Takahashi M, Furukawa K, Hirata K, Yuki N. Antecedent infections in Fisher syndrome: a common pathogenesis of molecular mimicry. Neurology. 2005. 64:1605–1611.
18. Aranyi Z, Kovacs T, Sipos I, Bereczki D. Miller Fisher syndrome: brief overview and update with a focus on electrophysiological findings. Eur J Neurol. 2012. 19:15–20.
19. Hsueh KC, Chou IC, Hsu CH, Kuo HT, Tsai FJ, Tsai CH. Miller Fisher syndrome possibly related to mycoplasma pneumoniae infection: report of one case. Acta Paediatr Taiwan. 2004. 45:168–170.
20. San-Juan OD, Martinez-Herrera JF, Garcia JM, Gonzalez-Aragon MF, Del Castillo-Calcaneo Jde D, Perez-Neri I. Miller fisher syndrome: 10 years' experience in a third-level center. Eur Neurol. 2009. 62:149–154.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download