Abstract
Sarcoidosis is a systemic granulomatous disease of unknown etiology that involves many organs, occasionally mimicking malignancy. We herein report a 50-yr-old woman of muscular sarcoidosis of chronic myopathic type, manifested by hypercalcemia and muscle wasting. Besides insignificant hilar lymphadenopathy, her sarcoidosis was confined to generalized atrophic muscles and therefore, F-18 FDG PET/CT alone among conventional imaging studies provided diagnostic clues for the non-parathyroid-related hypercalcemia. On follow-up PET/CT during low-dose steroid treatment, FDG uptake in the muscles disappeared whereas that in the hilar lymph nodes remained. PET/CT may be useful in the evaluation of unexpected disease extent and monitoring treatment response in suspected or known sarcoidosis patients.
Sarcoidosis is a systemic granulomatous disease of unknown etiology that involves many organs. The manifestations vary by involved site and grade, occasionally mimicking malignancy. Isolated muscular involvement is known as common in sarcoidosis patients, but painless or impalpable cases may be undiagnosed (1, 2). We report a case of generalized muscular sarcoidosis detected only by fluorine-18 fluorodeoxyglucose (F-18 FDG) positron emission tomography (PET)/computed tomography (CT) performed during the evaluation of non-parathyroid-related hypercalcemia.
In November 2009, a 50-yr-old female was admitted to our hospital with idiopathic transient pulmonary edema. Her weight was 49 kg, creatinine clearance 33.8 mL/min and calcium 9.9 mg/dL. Her echocardiogram showed mild diastolic dysfunction with ejection fraction of 55%. Her pulmonary function was moderately restricted with 51% of forced vital capacity. Her pulmonary edema was rapidly improved with diuretics therapy. In August 2010, she was re-admitted with hypercalcemia. She had underlying diabetes for 20 yr. Her diabetes was complicated with proliferative retinopathy, chronic renal impairment, and a chronic foot ulcer. At the time of her recent admission, her weight was 37 kg and she appeared chronically ill and emaciated. She reported 2 weeks of general weakness. We could not find any abnormal mass or tenderness except generalized muscle atrophy at the beginning. Creatinine clearance was 19.4 mL/min, calcium 14.5 mg/dL, phosphate 5.0 mg/dL, and ionized calcium 1.78 mM/L. Her 24-hr urine calcium level was 186.2 mg/day, and intact parathyroid hormone (PTH) of 15.77 pg/mL. These ambiguous laboratory findings prompted further studies of non-parathyroid-related hypercalcemia. Her 25-hydroxy Vitamin D level was 21.3 ng/mL, 1,25-dihydroxy Vitamin D was 43.1 pg/mL (normal, 25.1-66.1), and parathyroid hormone related peptide (PTHrP) was under 1.1 pM/L. The thyroid hormone level, serum electrophoresis and tumor markers were normal. The angiotensin-converting enzyme (ACE) level was elevated to 353.1 IU/L (normal, 20-70), and muscle enzymes (CPK/LDH/AST) were normal. The chest radiograph, neck ultrasound, and whole body bone scan were unremarkable. F-18 FDG PET/CT was performed for the evaluation of possible hidden malignancy. The PET/CT showed increased uptake of small lymph nodes in the subcarina and both hila. In addition, multiple streaky and dotted muscular uptakes were noted along whole body including the back and extremities (Fig. 1). We reexamined her whole body and found a 2 cm-sized soft and non-tender mass in the left gastrocnemius muscle. Excisional biopsy was performed of the mass, and the microscopic finding demonstrated non-necrotizing granulomas with multinucleated giant cells and without acid-fast bacilli or fungi (Fig. 2) consistent with muscular sarcoidosis. Imaging studies also revealed gallstones and bilateral renal stones, and we performed extracorporeal shock wave lithotripsy of the ureteral stone successfully. However, some renal stones remained.
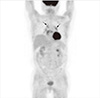
Acute severe hypercalcemia was relieved temporarily by hydration and furosemide. Prednisolone was started with 20 mg per day and then rapidly tapered to 5 mg per day within 1 week. The serum calcium level was normalized and maintained with prednisolone 5 mg a day, but ACE level continued to fluctuate. Her follow-up clinical data were laid out in the Table 1. Her glucose levels were controlled with insulin, and her weight increased to 50 kg with muscle gain. On follow-up PET/CT at one and half year later, muscular uptake was no longer seen. However, increased uptake in the mediastinal and hilar lymph nodes persisted (Fig. 3).
Muscular involvement is a common, usually asymptomatic feature of sarcoidosis. The presence of noncaseating epithelioid cell granulomas was detected in 50%-80% of sarcoidosis patients on random muscle biopsy (3). However, symptomatic muscular sarcoidosis is noted in only 0.5%-2.3%, and is more common in patients with extensive systemic involvement. There are three clinical subtypes of muscular sarcoidosis: acute myositic, chronic myopathic, and palpable nodular (1, 4, 5). The acute myositic type is accompanied by myalgia, fevers and elevated muscle enzymes, whereas the chronic myopathic type shows muscle atrophy and muscle weakness (6). The palpable nodular type presents as non-tender muscular masses requiring differential diagnosis of nodular muscular diseases (5).
Diagnosis of sarcoidosis is based on the combination of clinical, radiologic and histologic findings. Because of its unknown etiology, sarcoidosis remains a diagnosis of exclusion. The differential diagnosis includes neoplastic diseases such as lymphoma and various granulomatous diseases (7). Clinical symptoms and signs vary based on the organ affected.
No laboratory tests are diagnostic of sarcoidosis. The ACE level is elevated in approximately two-thirds of patients with sarcoidosis, but this finding is not diagnostic. Hypercalcemia is manifested in 5%-11% of patients with sarcoidosis because of overproduction of active Vitamin D by macrophages in the sarcoid granuloma as the enzyme 1α-hydroxylase is activated for immune modulation without feedback regulation (8). According to another hypothesis, hypovitaminosis D may causes sarcoidosis (9). In contrast to parathyroid- or PTHrP-related hypercalcemia, active Vitamin D-induced hypercalcemia usually accompanies hyperphosphatemia as noted in this case. Hypercalcemia can induce hypercalciuria and nephrolithiasis, and even renal function impairment. In this patient, in addition to underlying diabetic nephropathy, renal function was aggravated transiently due to hypercalcemia and ureteral stone-related obstructive uropathy. However, the patient's laboratory findings were not diagnostic of hypervitaminosis D. Although her PTH level was not the cause of this patient's hypercalcemia, it was not appropriately depressed. The accompanying hyperphosphatemia might stimulate the parathyroid gland in this case. The 1,25-dihydroxy Vitamin D level was in a relatively high normal range compared with the 25-hydroxy Vitamin D and the serum calcium levels. The diagnostic clue for hypercalcemia among her laboratory findings was only high ACE level.
Imaging abnormalities are commonly noted in sarcoidosis patients. Most of patients with sarcoidosis demonstrate bilateral hilar lymphadenopathy on chest radiograph or CT (7, 10, 11). In this patient, enlargement of lymph nodes in the mediastinum and hila were subtle. She had sarcopenia and general weakness but did not manifest myalgia. Her muscle enzymes were also normal. If we first performed CT in our search for a hidden malignancy, we would not have discovered the presence of muscle sarcoidosis, and therefore the cause of her hypercalcemia. This type of muscular sarcoidosis seems to be chronic myopathy with muscle wasting.
FDG PET/CT has been used worldwide for staging and restaging various malignancies. Because FDG uptake reflects tissue metabolism, PET/CT can detect some infectious and inflammatory diseases as well as malignancy. Recently, PET/CT has been used in evaluation of fever of unknown origin and hypercalcemia (12-14). Hypercalcemia is a common clinical finding, mainly related to hyperparathyroidism and malignancy. In hypercalcemic patients with unremarkable PTH level, anatomical and functional imaging studies are necessary to investigate the possibility of occult malignancy (14). Sarcoidosis is a well-known granulomatous disease with increased FDG uptake (15).
Traditionally, gallium-67 scan has been used in the detection and evaluation of extent of sarcoidosis. Recent study reported that FDG PET demonstrates higher sensitivity in detection of sarcoidosis involvement compared to the gallium-67 scan (79% vs 58%). Another study reported that FDG PET contributes to a better evaluation of sarcoidosis especially in extrapulmonary involvement. In addition, FDG PET requires a shorter imaging time and delivers a lower radiation dose compared to the gallium-67 scan (16-18). Magnetic resonance imaging (MRI) has been used in the diagnosis of sarcoidosis. In muscular sarcoidosis patients, MRI is useful to assess degree of invasion as well as extent of disease. However, any type of muscular sarcoidosis may not be detected in MRI and regional MRI is difficult to apply to a patient with uncertain symptom that is not localized (19). Because the whole body is evaluated in FDG PET, location of involvement can be discovered, and biopsy site can be determined in suspected sarcoidosis patients (4, 16, 17). In our patient, the left lower leg was selected as a proper biopsy site because this lesion was the only palpable nodule, was located in a relatively superficial area and showed intense FDG uptake in PET/CT.
FDG PET is useful for monitoring treatment response in known sarcoidosis patients. Several reports have demonstrated that changes in FDG uptake before and after treatment correlate with clinical symptoms and laboratory tests (4, 17, 20). Braum et al. reported a patient with disease progression that showed increased FDG uptake in post-treatment PET (16). In our patient, abnormal muscular uptake was no longer visualized in follow-up PET/CT, but increased uptake in mediastinal and bilateral hilar lymph nodes persisted. Although clinical improvement was maintained with continuity of low dose steroid therapy, uptake in lymph nodes may be related to the remaining activity of the disease.
In summary, we report a case of a woman who presented with hypercalcemia and renal stones and was found to have atypical sarcoidosis mainly involving generalized skeletal muscles with minimal hilar lymph node involvement. FDG PET/CT secured the diagnosis. In conclusion, FDG PET/CT can be useful to evaluate unexpected disease extent and to monitor treatment response in suspected or known sarcoidosis patients.
Figures and Tables
Fig. 1
F-18 FDG PET/CT findings. In maximum intensity projection (MIP) (A) and coronal (B, C) images of PET/CT, increased uptake was noted in mediastinal and bilateral hilar lymph nodes (arrows). In addition, multiple streaky and dotted muscular uptakes were noted along whole body. Nodular uptake was seen in left lower lateral leg (arrowhead) in MIP (A) and transaxial (D) images, and excisional biopsy was performed in this mass.
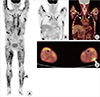
Fig. 2
Histologic result. Biopsy specimen was obtained from muscular mass of left lower leg. Microscopic findings (hematoxylin-eosin stain) demonstrated non-necrotizing granulomas (arrows) with multinucleated giant cell (arrowhead) and without acid-fast bacilli or fungi. The histology was consistent with muscular sarcoidosis.

Fig. 3
Follow-up PET/CT finding. In MIP image, previously noted multiple muscular uptakes were no longer seen. However, increased uptake in mediastinal and bilateral hilar lymph nodes persisted (arrows).
References
1. Vardhanabhuti V, Venkatanarasimha N, Bhatnagar G, Maviki M, Iyengar S, Adams WM, Suresh P. Extra-pulmonary manifestations of sarcoidosis. Clin Radiol. 2012; 67:263–276.
2. Spagnolo P, Luppi F, Roversi P, Cerri S, Fabbri LM, Richeldi L. Sarcoidosis: challenging diagnostic aspects of an old disease. Am J Med. 2012; 125:118–125.
3. Silverstein A, Siltzbach LE. Muscle involvement in sarcoidosis: asymptomatic, myositis, and myopathy. Arch Neurol. 1969; 21:235–241.
4. Marie I, Lahaxe L, Vera P, Edet-Samson A. Follow-up of muscular sarcoidosis using fluorodeoxyglucose positron emission tomography. QJM. 2010; 103:1000–1002.
5. Tohme-Noun C, Le Breton C, Sobotka A, Boumenir ZE, Milleron B, Carette MF, Khalil A. Imaging findings in three cases of the nodular type of muscular sarcoidosis. AJR Am J Roentgenol. 2004; 183:995–999.
6. Costabel U. Skeletal muscle weakness, fatigue and sarcoidosis. Thorax. 2005; 60:1–2.
7. Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997; 336:1224–1234.
8. Conron M, Young C, Beynon HL. Calcium metabolism in sarcoidosis and its clinical implications. Rheumatology (Oxford). 2000; 39:707–713.
9. Richmond BW, Drake WP. Vitamin D, innate immunity, and sarcoidosis granulomatous inflammation: insights from mycobacterial research. Curr Opin Pulm Med. 2010; 16:461–464.
10. Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet. 2003; 361:1111–1118.
11. Studdy PR, Lapworth R, Bird R. Angiotensin-converting enzyme and its clinical significance: a review. J Clin Pathol. 1983; 36:938–947.
12. Meller J, Sahlmann CO, Scheel AK. 18F-FDG PET and PET/CT in fever of unknown origin. J Nucl Med. 2007; 48:35–45.
13. Solav SV. FDG PET/CT in evaluation of pyrexia of unknown origin. Clin Nucl Med. 2011; 36:e81–e86.
14. Cecchin D, Motta R, Zucchetta P, Bui F, Basso SM, Lumachi F. Imaging studies in hypercalcemia. Curr Med Chem. 2011; 18:3485–3493.
15. Culverwell AD, Scarsbrook AF, Chowdhury FU. False-positive uptake on 2-[18F]-fluoro-2-deoxy-D-glucose (FDG) positron-emission tomography/computed tomography (PET/CT) in oncological imaging. Clin Radiol. 2011; 66:366–382.
16. Braun JJ, Kessler R, Constantinesco A, Imperiale A. 18F-FDG PET/CT in sarcoidosis management: review and report of 20 cases. Eur J Nucl Med Mol Imaging. 2008; 35:1537–1543.
17. Aide N, Benayoun M, Kerrou K, Khalil A, Cadranel J, Talbot JN. Impact of [18F]-fluorodeoxyglucose ([18F]-FDG) imaging in sarcoidosis: unsuspected neurosarcoidosis discovered by [18F]-FDG PET and early metabolic response to corticosteroid therapy. Br J Radiol. 2007; 80:e67–e71.
18. Nishiyama Y, Yamamoto Y, Fukunaga K, Takinami H, Iwado Y, Satoh K, Ohkawa M. Comparative evaluation of 18F-FDG PET and 67Ga scintigraphy in patients with sarcoidosis. J Nucl Med. 2006; 47:1571–1576.
19. Sohn HS, Kim EN. A case of muscular sarcoidosis diagnosed by gallium-67 scintigraphy and magnetic resonance imaging. Korean J Nucl Med. 1999; 33:543–548.
20. Lee JH, Lim YJ, Lee S, Joo KB, Choi YY, Park CK, Lee YH. Early-onset childhood sarcoidosis with incidental multiple enchondromatosis. J Korean Med Sci. 2012; 27:96–100.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download