Abstract
Amiloride and benzamil showed antinocicepitve effects in several pain models through the inhibition of acid sensing ion channels (ASICs). However, their role in neuropathic pain has not been investigated. In this study, we investigated the effect of the intrathecal amiloride and benzamil in neuropathic pain model, and also examined the role of ASICs on modulation of neuropathic pain. Neuropathic pain was induced by L4-5 spinal nerve ligation in male Sprague-Dawley rats weighing 100-120 g, and intrathecal catheterization was performed for drug administration. The effects of amiloride and benzamil were measured by the paw-withdrawal threshold to a mechanical stimulus using the up and down method. The expression of ASICs in the spinal cord dorsal horn was also analyzed by RT-PCR. Intrathecal amiloride and benzamil significantly increased the paw withdrawal threshold in spinal nerve-ligated rats (87%±12% and 76%±14%, P=0.007 and 0.012 vs vehicle, respectively). Spinal nerve ligation increased the expression of ASIC3 in the spinal cord dorsal horn (P=0.01), and this increase was inhibited by both amiloride and benzamil (P<0.001 in both). In conclusion, intrathecal amiloride and benzamil display antinociceptive effects in the rat spinal nerve ligation model suggesting they may present an alternative pharmacological tool in the management of neuropathic pain at the spinal level.
Neuropathic pain is a complex, chronic pain state typically accompanied by disease of the somatosensory system that is associated with abnormal sensations, such as allodynia, hyperalgesia, and spontaneous pain (1). These abnormal sensations may greatly influence on the quality of life, cause problems with mood and sleep, and cause patients to withdraw from social activities. Moreover, commonly used analgesics are often inadequate in treating neuropathic pain. Present pharmacologic therapy is not enough effective in approximate 50% of neuropathic pain patients (2). Thus, there is a continuing need for the development of effective analgesic therapies for neuropathic pain.
Amiloride and its potent analog benzamil are well known diuretics used in management of congestive heart disease or hypertension. They are also commercially available inhibitors for acid sensing ion channels (ASICs). There are several reports that amiloride or benzamil showed antinociceptive effects in animal (3, 4) or human (5) through the inhibition of ASICs.
ASICs are sodium-selective ion channels that are sensitive to protons (6). To date, six subunits of ASICs have been identified: 1a, 1b, 2a, 2b, 3, and 4 (7). Some of these ASICs are expressed in the spinal cord (8, 9). Moreover, intrathecal ASIC1a inhibitors showed anti-nociceptive effects in acute and chronic pain states (10, 11). These findings suggest that spinal ASICs may play an important role in the development of nociception, but there is limited data concerning their role in neuropathic pain in the spinal cord.
The purpose of the present study was to investigate the effects of intrathecal amiloride and benzamil in rat spinal nerve ligation-induced neuropathic pain. We also examined the role of ASICs in the modulation of neuropathic pain at the spinal level.
Animal handling and experimental procedures were approved by the institutional animal care committee of Chonnam National University and followed the guidelines on ethical standards for the investigation of experimental pain in animals (12). Male Sprague-Dawley rats, weighing 100-120 g, were used in all experiments. Animals were acclimatized to the laboratory environment for more than 1 week prior to the study. During this period, animals were housed with free access to a standard rat diet and tap water in a room under a 12/12-h light/dark cycle.
Neuropathic pain in rats was evoked by spinal nerve ligation, as described previously (13). During sevoflurane anesthesia, the left L5 and L6 spinal nerves were located adjacent to the vertebral column and ligated tightly to the dorsal root ganglia using a 6-0 silk suture. Animals showing mechanical allodynia were considered to be developing neuropathic pain (i.e., a paw-flinch behavioral response to the application of a bending force less than 4 g) (14). At 5 days after spinal nerve ligation (SNL), a polyethylene-10 tube was inserted through an incision in the atlanto-occipital membrane to the subarachnoid space (15). Rats showing neurological deficits following catheterization were immediately euthanized with an overdose of volatile anesthetics. Experiments were performed 5 days after catheterization allowing time for recovery.
The following drugs were used in this study: amiloride hydrochloride (Tocris Cookson Ltd., Bristol, UK) and benzamil hydrochloride (Sigma Aldrich Co., St. Louis, MO, USA). Amiloride hydrochloride was dissolved in dimethyl sulfoxide (DMSO), and diluted in saline; benzamil hydrochloride was dissolved in methanol, and diluted with saline. Both agents were administered intrathecally using a hand-driven, gear-operated syringe pump (Model 1750LT Threaded Plunger Syringe, Hamilton Co., Reno, NV, USA). All drugs were delivered in a volume of 10 µL, and an additional 10 µL of normal saline was used to flush the catheter.
The paw withdrawal threshold (PWT) in response to mechanical stimulation was measured using the up and down method (16) by applying calibrated von Frey filaments (Stoelting, Wood Dale, IL, USA). The cut-off value was determined as 15 g if the rat did not show any withdrawal or licking response. Rats that did not show allodynia (less than 4 g) were excluded from the study.
On the day of the experiment, rats were allocated into experimental and control groups for the tested drugs. Control groups were performed using intrathecal DMSO (n=5) or methanol (n=5) according to the solvent used for the tested drugs. All experiments were performed by investigators blinded to the treatment. To assess the effects of both drugs, increasing doses of amiloride (1, 3, 10 µg in 10 µL, n=5-7) or benzamil (3, 10, 30 µg in 10 µL, n=5-7) were investigated. The withdrawal threshold was measured prior to spinal nerve ligation and was regarded as pre-ligated threshold. The withdrawal threshold was measured immediately prior to intrathecal drug delivery and was regarded as a post-ligated baseline threshold. The withdrawal threshold was determined at 15, 30, 60, 90, 120, 150, and 180 min following intrathecal administration of the experimental drugs.
For the purpose of examining the behavioral changes by amiloride and benzamil, the highest dose of each drug was administered intrathecally to 10 additional rats. Motor function was assessed by the righting reflex and placing-stepping reflex. The former was evaluated by placing the rat horizontally with its back on the table, which gives rise to an immediate coordinated twisting of the body to an upright position. The latter was evoked by drawing the dorsum of either hind paw across the edge of the table. Typically the rats attempt to place the paw into a position to walk. The pinna reflex and corneal reflex were also evaluated and judged as present or absent.
To determine the expression of ASIC subtype in the spinal cord, 20 additional rats (5 naive, 5 spinal nerve ligated, 5 amiloride treated, and 5 benzamil treated rats after SNL) were used for reverse transcriptase polymerase chain reaction (RT-PCR). On the 10th day after spinal nerve ligation, the spinal cord was extracted after decapitation under deep sevoflurane anesthesia, and the spinal dorsal horn obtained from the ipsilateral L4-5 lumbar spinal enlargement. Total RNA was extracted from the lumbar spinal cord with RNAiso Plus (Takara Bio, Otsu, Japan) according to the manufacturer's instructions. The yield of the RNA was determined by measuring the absorbance at 260 and 280 nm. RT-PCR was performed using a PrimeScript™ RT-PCR Kit (Takara Bio) according to the manufacturer's protocol (Table 1). Total RNA (1 µg) was reverse-transcribed for 60 min at 42℃, and PCR amplification was performed in 34 cycles of denaturation (94℃ for 30 sec), annealing (Table 1 for subunit-specific temperatures), and extension (72℃ for 2 min). The PCR products were separated on 1.2% agarose gels and made visible by ethidium bromide staining. The intensity of the bands was measured by densitometry, and the relative values of the ASIC subunits to the β-actin band were calculated.
Data are expressed as the means±SEM. Time response data are presented as the paw withdrawal threshold to mechanical stimulation, while the dose-response data are presented as the percent of the maximum possible effect (%MPE). The withdrawal threshold data from von Frey filament testing were converted to %MPE, according to the formula: %MPE=([post-drug threshold - post-ligated baseline threshold] / [cutoff threshold - post-ligated baseline threshold])×100. To analyze the 50% probability, PWT data and dose-responsiveness, a one-way analysis of variance with Scheffe multiple comparison test was used. ED50 and its 95% confidence interval (CI) were calculated using the method reported by Tallarida (17). P values of<0.05 were considered to indicate statistical significance.
Intrathecal amiloride and benzamil did not affect motor function, as assessed by the righting reflex and placing-stepping reflex. The pinna or corneal reflexes were also not affected.
Spinal nerve ligation resulted in a significant lowering the PWTs in injured site. This characteristic mechanical allodynia appeared after nerve ligation and persisted for 21 days, as was obviously observed in our previous study (18).
The baseline threshold after spinal cord ligation did not differ among the groups. While intrathecal DMSO or methanol had no effect, intrathecal amiloride and benzamil significantly increased the PWTs (87%±12% and 76%±14%, P=0.007 and 0.012 vs vehicle, respectively; Fig. 1 and 2). The ED50 values of amiloride and benzamil were 1.95 µg (95% CI, 0.38-10.01 µg) and 7.47 µg (95% CI, 4.3-12.98 µg), respectively.
RT-PCR analysis showed the presence of ASIC subunits in the spinal cord dorsal horn of naive rats. Following spinal nerve ligation, increased expression of the mRNA levels of spinal ASIC3 was observed, compared to those of naive rats (P=0.01; Fig. 3). Intrathecal amiloride and benzamil inhibited the increase of ASIC3 expression (P<0.001 in both; Fig. 4).
Neuropathic pain arises as a direct consequence of damage to the peripheral or central somatosensory system (1). Despite intensive investigation, the detailed mechanisms underlying neuropathic pain remain unclear. Moreover, the current therapeutic approaches to the management of neuropathic pain are limited, and continued use of some therapeutics can lead to a variety of adverse events. Thus, further studies are required to develop safer and more effective pain therapeutics.
In our present study, we demonstrated that intrathecal administration of amiloride and benzamil attenuated mechanical allodynia induced by spinal nerve ligation. Amiloride is a common blocker for all members of the degenerin/epithelial sodium channel (DEG/ENaC) family (19). Acid sensing ion channels (ASICs) are voltage-insensitive cationic channels belonging to DEG/ENaC family (20). In particular, experimental evidence has supported the involvement of ASICs in nociceptive processing (6). Peripheral ASICs are involved in the detection and transmission of numerous inflammatory or mechanical pain induced stimuli including cutaneous somatic pain (21), deep somatic pain (22) and visceral pain (23). Relevant to the peripheral nervous system, amiloride has been shown to display anti-nociceptive effects in acid-induced pain (21). Additionally, increased expression of ASICs was observed in the spinal cord following peripheral inflammation (9, 11).
However, there was limited data for involvement of ASICs in neuropathic pain. An intrathecal ASIC1a blocker reversed thermal hyperalgesia and tactile allodynia induced by peripheral nerve injury and vincristine-induced neuropathy (10). Furthermore, it has been reported that various subtypes of ASICs are expressed in the dorsal horn of the spinal cord (8, 9).
Contrary to our expectation, in our study, there is no change of ASIC1a expression in the spinal cord after spinal cord ligation. However, in the previous study (10), they examined the effect of the ASIC1a antagonist (psalmotoxin 1) without measuring the change of ASICs expression, and they suggested the endogenous opioid mechanisms related to the ASIC1a channel. So, it is thought that more studies will be required to confirm the function of ASIC1a in neuropathic pain.
In our study, all subunits of ASICs except ASIC1b were expressed to similar degree in rat spinal cord. In contrast to our findings, Wu et al. (9) reported a lack of ASIC3 in mouse spinal cord. In the study of Baron et al. (24), even though ASIC3 was expressed, its quantity was negligible. Moreover, there was no expression and alteration of ASIC3 in rat spinal cord in the inflammatory pain model (25). However, there was a report that demonstrated the expression of ASIC3 in mouse spinal cord, which used a different ASIC3 primer for RT-PCR (26). We used this ASIC3 primer, and this might be the reason why our study showed an increased amount of ASIC3.
In our study, there was a significant increase in the expression of ASIC3 in the rat spinal cord dorsal horn after spinal cord ligation, and this increase was significantly blocked by amiloride and benzamil. Among the ASICs, ASIC3 channel is known to regulate the mechanotransduction, nociceptive signaling (27), and mechanical hyperalgesia after inflammation (28, 29). Moreover, interfering with ASIC3 may be a beneficial target for arthritis pain (30).
However, another study using genetic knockout mice showed that ASIC3 did not alter the development or maintenance of mechanical hyperalgesia following spinal nerve ligation in rats, suggesting their lack of involvement in neuropathic pain (31). Therefore, the role of the spinal ASIC3 in the neuropathic pain is still ambiguous. But based on our data, it is conceivable that ASIC3 may be involved in development of mechanical allodynia and the inhibition of the spinal ASIC3 may be therapeutic option in neuropathic pain management.
In our study, we did not use specific ASICs inhibitors because of lack of specific inhibitors. Amiloride and benzamil are non-specific ASIC inhibitors and have been shown to also inhibit the cell surface Na+/Ca2+ exchangers and voltage dependent Ca2+ channels at similar concentrations (32). Na+/Ca2+ exchangers are anti-porter membrane proteins that decrease intracellular calcium levels through active transport. Several pathological states, including ischemia, ATP depletion and membrane depolarization, activate Na+/Ca2+ exchangers. Furthermore, several studies have suggested that the activation of Na+/Ca2+ exchangers exacerbates mechanical or ischemic nerve injuries (33, 34). It has been reported that intraperitoneal injection of amiloride and pralidoxime (a Na+/Ca2+ exchanger inhibitor) attenuated the sciatic nerve ligation-induced decrease in the PWT (35). T-type Ca2+ channels in neurons lower the action potential threshold, promoting burst firing and an enhancement of synaptic excitation (36). Recent data suggests that both central and peripheral T-type Ca2+ channels are involved in neuropathic pain; indeed, peripheral T-type Ca2+ channel inhibition has known anti-nociceptive effects in response to neuropathic pain (37). Furthermore, chronic dorsal root ganglion compression increased the expression of T-type Ca2+ channels in the spinal cord, and intrathecal injection of T-type Ca2+ channel inhibitors significantly reduced neuropathic pain behavior (38). Taking this into account, we cannot rule out a contribution of Na+/Ca2+ exchanger and/or T-type Ca2+ channel inhibition in the spinal cord to the antinociceptive effects of intrathecal amiloride and benzamil. In addition, recently it has been reported that nonproton ligand sensor existing in the ASICs causes persistent activations of ASICs (39, 40). Thus, nonproton ligand sensor may contribute to the effect of amiloride and benzamil.
In conclusion, intrathecal amiloride and benzamil display antinociceptive effects in the rat spinal nerve ligation model, suggesting they may present a candidate therapy for the management of neuropathic pain at the spinal level. Additionally, spinal nerve ligation increases the expression of ASIC3 in the spinal cord and this increase is blocked by amirolide and benzamil, suggesting ASIC3 may contribute to the antinociceptive effects of amiloride and benzamil in neuropathic pain.
Figures and Tables
Fig. 1
Effects of intrathecal amiloride on the hindpaw withdrawal response after spinal nerve ligation. Data are presented as the withdrawal threshold (g) or the percentage of the maximal possible effect (%MPE). Each line or bar represents the means±SEM of 5-7 rats. Baseline data (BL) were measured immediately before intrathecal delivery of drugs or vehicle. Intrathecal amiloride produced an increase of the withdrawal threshold. *P<0.05 vs vehicle group as determined by one-way analysis of variance with Scheffe's post hoc test.

Fig. 2
Effects of intrathecal benzamil on the hindpaw withdrawal response after spinal nerve ligation. Data are presented as the withdrawal threshold (g) or the percentage of the maximal possible effect (%MPE). Each line or bar represents the means±SEM of 5-7 rats. Baseline data (BL) were measured immediately before intrathecal delivery of drugs or vehicle. Intrathecal benzamil produced an increase of the withdrawal threshold. *P<0.05 vs vehicle group as determined by one-way analysis of variance with Scheffe's post hoc test.

Fig. 3
Acid-sensing ion channel (ASIC) expression in the rat spinal dorsal horn (n=5, each group). Data are presented as the means±SEM. All subtypes of ASIC except ASIC2 are expressed in the rat spinal dorsal horn. The expression of ASIC3 was increased in the spinal dorsal horn following spinal cord ligation. *P<0.01 vs naive group as determined by one-way analysis of variance with Scheffe's post hoc test. SNL, spinal nerve-ligated group.
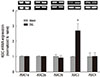
Fig. 4
Effects of Amiloride and benzamil on ASIC3 expression in the rat spinal dorsal horn. Data are presented as the means±SEM. Amiloride and benzamil significantly inhibited the increase in ASIC3 expression after spinal cord ligation. *P<0.001 vs spinal nerve-ligated group as determined by one-way analysis of variance with Scheffe's post hoc test. SNL, spinal nerve-ligated group.

References
1. Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008; 70:1630–1635.
2. Wallace JM. Update on pharmacotherapy guidelines for treatment of neuropathic pain. Curr Pain Headache Rep. 2007; 11:208–214.
3. Rocha-González HI, Castañeda-Corral G, Araiza-Saldaña CI, Ambriz-Tututi M, Caram-Salas NL, Torres-López JE, Murbartián J, Granados-Soto V. Identification of the Na+/H+ exchanger 1 in dorsal root ganglion and spinal cord: its possible role in inflammatory nociception. Neuroscience. 2009; 160:156–164.
4. Page AJ, Brierley SM, Martin CM, Hughes PA, Blackshaw LA. Acid sensing ion channels 2 and 3 are required for inhibition of visceral nociceptors by benzamil. Pain. 2007; 133:150–160.
5. Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest. 2002; 110:1185–1190.
6. Colombo E, Francisconi S, Faravelli L, Izzo E, Pevarello P. Ion channel blockers for the treatment of neuropathic pain. Future Med Chem. 2010; 2:803–842.
7. Krishtal O. The ASICs: signaling molecules? modulators? Trends Neurosci. 2003; 26:477–483.
8. Dubé GR, Elagoz A, Mangat H. Acid sensing ion channels and acid nociception. Curr Pharm Des. 2009; 15:1750–1766.
9. Wu LJ, Duan B, Mei YD, Gao J, Chen JG, Zhuo M, Xu L, Wu M, Xu TL. Characterization of acid-sensing ion channels in dorsal horn neurons of rat spinal cord. J Biol Chem. 2004; 279:43716–43724.
10. Mazzuca M, Heurteaux C, Alloui A, Diochot S, Baron A, Voilley N, Blondeau N, Escoubas P, Gélot A, Cupo A, et al. A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nat Neurosci. 2007; 10:943–945.
11. Duan B, Wu LJ, Yu YQ, Ding Y, Jing L, Xu L, Chen J, Xu TL. Upregulation of acid-sensing ion channel ASIC1a in spinal dorsal horn neurons contributes to inflammatory pain hypersensitivity. J Neurosci. 2007; 27:11139–11148.
12. Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983; 16:109–110.
13. Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992; 50:355–363.
14. Calcutt NA, Jorge MC, Yaksh TL, Chaplan SR. Tactile allodynia and formalin hyperalgesia in streptozotocin-diabetic rats: effects of insulin, aldose reductase inhibition and lidocaine. Pain. 1996; 68:293–299.
15. Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976; 17:1031–1036.
16. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63.
17. Tallarida RJ. Drug synergism and dose-effect data analysis. New York: Chapman & Hall/CRC;2000.
18. Lee HG, Park SK, Yoon MH. Potentiation of morphine antiallodynic efficacy by ACPT-III, a group III metabotropic glutamate receptor agonist, in rat spinal nerve ligation-induced neuropathic pain. Pharmacol Biochem Behav. 2010; 96:108–113.
19. Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002; 82:735–767.
20. Lingueglia E. Acid-sensing ion channels in sensory perception. J Biol Chem. 2007; 282:17325–17329.
21. Rocha-González HI, Herrejon-Abreu EB, López-Santillán FJ, García-López BE, Murbartián J, Granados-Soto V. Acid increases inflammatory pain in rats: effect of local peripheral ASICs inhibitors. Eur J Pharmacol. 2009; 603:56–61.
22. Walder RY, Rasmussen LA, Rainier JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 play different roles in the development of hyperalgesia after inflammatory muscle injury. J Pain. 2010; 11:210–218.
23. Wultsch T, Painsipp E, Shahbazian A, Mitrovic M, Edelsbrunner M, Lazdunski M, Waldmann R, Holzer P. Deletion of the acid-sensing ion channel ASIC3 prevents gastritis-induced acid hyperresponsiveness of the stomach-brainstem axis. Pain. 2008; 134:245–253.
24. Baron A, Voilley N, Lazdunski M, Lingueglia E. Acid sensing ion channels in dorsal spinal cord neurons. J Neurosci. 2008; 28:1498–1508.
25. Matricon J, Gelot A, Etienne M, Lazdunski M, Muller E, Ardid D. Spinal cord plasticity and acid-sensing ion channels involvement in a rodent model of irritable bowel syndrome. Eur J Pain. 2011; 15:335–343.
26. Hruska-Hageman AM, Benson CJ, Leonard AS, Price MP, Welsh MJ. PSD-95 and Lin-7b interact with acid-sensing ion channel-3 and have opposite effects on H+- gated current. J Biol Chem. 2004; 279:46962–46968.
27. Deval E, Noël J, Lay N, Alloui A, Diochot S, Friend V, Jodar M, Lazdunski M, Lingueglia E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008; 27:3047–3055.
28. Ikeuchi M, Kolker SJ, Sluka KA. Acid-sensing ion channel 3 expression in mouse knee joint afferents and effects of carrageenan-induced arthritis. J Pain. 2009; 10:336–342.
29. Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007; 129:102–112.
30. Yuan FL, Chen FH, Lu WG, Li X. Acid-sensing ion channels 3: a potential therapeutic target for pain treatment in arthritis. Mol Biol Rep. 2010; 37:3233–3238.
31. Borzan J, Zhao C, Meyer RA, Raja SN. A role for acid-sensing ion channel 3, but not acid-sensing ion channel 2, in sensing dynamic mechanical stimuli. Anesthesiology. 2010; 113:647–654.
32. Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008; 153:S1–S209.
33. Craner MJ, Hains BC, Lo AC, Black JA, Waxman SG. Co-localization of sodium channel Nav1.6 and the sodium-calcium exchanger at sites of axonal injury in the spinal cord in EAE. Brain. 2004; 127:294–303.
34. Stys PK, Waxman SG, Ransom BR. Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na(+)-Ca2+ exchanger. J Neurosci. 1992; 12:430–439.
35. Muthuraman A, Jaggi AS, Singh N, Singh D. Ameliorative effects of amiloride and pralidoxime in chronic constriction injury and vincristine induced painful neuropathy in rats. Eur J Pharmacol. 2008; 587:104–111.
36. Jagodic MM, Pathirathna S, Joksovic PM, Lee W, Nelson MT, Naik AK, Su P, Jevtovic-Todorovic V, Todorovic SM. Upregulation of the T-type calcium current in small rat sensory neurons after chronic constrictive injury of the sciatic nerve. J Neurophysiol. 2008; 99:3151–3156.
37. Todorovic SM, Meyenburg A, Jevtovic-Todorovic V. Mechanical and thermal antinociception in rats following systemic administration of mibefradil, a T-type calcium channel blocker. Brain Res. 2002; 951:336–340.
38. Wen XJ, Xu SY, Chen ZX, Yang CX, Liang H, Li H. The roles of T-type calcium channel in the development of neuropathic pain following chronic compression of rat dorsal root ganglia. Pharmacology. 2010; 85:295–300.
39. Li WG, Yu Y, Zhang ZD, Cao H, Xu TL. ASIC3 channels integrate agmatine and multiple inflammatory signals through the nonproton ligand sensing domain. Mol Pain. 2010; 6:88.
40. Yu Y, Chen Z, Li WG, Cao H, Feng EG, Yu F, Liu H, Jiang H, Xu TL. A nonproton ligand sensor in the acid-sensing ion channel. Neuron. 2010; 68:61–72.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download