Abstract
The aim of this study was to determine whether intra-amniotic infection/inflammation (IAI) was associated with subsequent ruptured membranes in women with preterm labor and intact membranes who had a clinically indicated amniocentesis. This retrospective cohort study included 237 consecutive women with preterm labor (20-34.6 weeks) who underwent amniocentesis. The clinical and laboratory parameters evaluated included demographic variables, gestational age, C-reactive protein (CRP) and amniotic fluid (AF) white blood cell, interleukin-6 (IL-6) and culture results. IAI was defined as a positive AF culture and/or an elevated AF IL-6 level (>2.6 ng/mL). The primary outcome was ruptured membranes in the absence of active labor occurring within 48 hours of amniocentesis. Preterm premature rupture of membranes subsequently developed in 10 (4.2%) women within 48 hr of amniocentesis. Multivariate analysis demonstrated that only IAI was independently associated with the ruptured membranes occurring within 48 hr of amniocentesis. In the predictive model based on variables assessed before amniocentesis, only CRP level was retained. IAI is an independent risk factor for subsequent ruptured membranes after clinically indicated amniocentesis in preterm labor. Prior to amniocentesis, measurement of serum CRP level can provide a risk assessment for the subsequent development of ruptured membranes after the procedure.
Intra-amniotic infection/inflammation has been implicated in the mechanism responsible for preterm labor and has been found in at least 20% of women in preterm labor and intact membranes (1-5). Importantly, recent studies indicated that maternal parenteral treatment with antibiotics can eradicate microbial invasion of amniotic cavity in women with preterm labor and this was associated with delivery at term in these patients whose successful treatment was documented by microbial studies (6-8). In addition, fetal maturity assessment by amniotic fluid (AF) analysis is used to enhance management decisions taking into consideration the benefits and risks of waiting for pulmonary maturity and continued exposure to hostile intra-uterine environment in pregnancies complicated by preterm labor. Therefore, amniocentesis may seem justified in cases with preterm labor to suspect intra-amniotic infection/inflammation, as causes for this condition.
Rupture of membranes is one of the immediate complications after amniocentesis in preterm labor (9), with an estimated risk of approximately 17% (10). Practitioner and their patients fear such an event after amniocentesis, to the extent that women may not opt for amniocentesis. However, it is unclear whether the rupture of membranes that occurs after an amniocentesis is always attributable to the procedure itself or simply represents the progression of a related event in the natural history of the disease process that results in spontaneous preterm delivery, especially if the initiator of preterm labor is an intra-amniotic infection/inflammation. Indeed, in asymptomatic women undergoing mid-trimester genetic amniocentesis, several studies have recently reported that isolation of microorganisms or marked elevations of matrix metalloproteinase-8 (MMP-8) in mid-trimester AF was highly associated with subsequent preterm premature rupture of membranes (PPROM) (11, 12) and supported the idea that disease progression is the cause. However, despite the urgent clinical and medico-legal needs for such information, there is a paucity of data on this issue for women with preterm labor undergoing amniocentesis.
The purpose of this study was to determine whether intra-amniotic infection/inflammation at the time of amniocentesis was associated with subsequent PPROM in women with preterm labor and intact membranes who had a clinically indicated amniocentesis.
We conducted a retrospective cohort study of all women who were admitted to the Seoul National University Bundang Hospital (Seongnam, Korea) from June 2003 to October 2011 with the diagnosis of preterm labor and intact membranes, and who underwent an amniocentesis. A detailed database of all obstetric patients admitted to the high-risk pregnancy unit at our institution has been maintained since 2003. Data at the time of amniocentesis (i.e., time of amniocentesis, maternal demographics, biochemical tests results in AF and in maternal blood, and other perinatal factors) were collected prospectively by means of an electronically-prepared Excel-based (Microsoft Corporation, Redmond, WA, USA) data collection tool. From this clinical database, patients were retrospectively considered for inclusion in our analysis. The inclusion criteria were: 1) singleton gestation; 2) transabdominal amniocentesis performed for the assessment of microbiologic and inflammatory status of the amniotic cavity and/or fetal lung maturity; 3) maternal blood drawn for the determination of white blood cell (WBC) and C-reactive protein (CRP) concentrations at amniocentesis; 4) a live fetus with a gestational age between 20 and 34 weeks; 5) cervical dilatation of ≤3 cm by digital examination; 6) absence of a major fetal congenital anomaly. Amniocentesis for retrieval of AF was recommended to all patients who were admitted with the diagnosis of preterm labor and intact membranes at our institution. Throughout the study period, maternal WBC count and serum CRP level were routinely determined at the time of amniocentesis on all women with preterm labor and intact membranes. Medical records regarding the presence or absence of ruptured membranes within 48 hr and 7 days after amniocentesis were reviewed by one of the authors who was unaware of the laboratory test results.
AF was obtained by transabdominal amniocentesis under ultrasonographic guidance. Maternal fetal medicine faculties or fellows performed all of the amniocenteses in an inpatient setting with an obstetric resident assisting. All procedures took place on the labor and delivery floor and all were performed using 22-gauge needles. After amniocentesis, the women underwent external cardiotocography to document fetal well-being and to obtain information on contractions. Microbiologic evaluation of the amniotic cavity included cultures for aerobic and anaerobic bacteria, and mycoplasmas (Ureaplasma urealyticum and Mycoplasma hominis). For aerobic and anaerobic cultures, amniotic fluid was inoculated into resin-containing BACTEC Plus Aerobic/F and Anaerobic/F culture bottles (Becton Dickinson, Sparks, MD, USA). The bottles were then incubated in a BACTEC FX (Becton Dickinson) automated culture system until an alarm signal indicated positivity. For culture and identification of Ureaplasma urealyticum and Mycoplasma hominis, the specimens were also inoculated into Mycofast Evolution 2 (Elitech Microbiology, Signes, France) according to the manufacturer's instructions. An aliquot of AF was transferred to the hematology laboratory and examined in a hemocytometer chamber for the presence of WBCs. The absolute WBC count was calculated by multiplying the area examined by a factor of 10 per area and was expressed as the number of cells per cubic millimeter. AF that was not required for clinical assessment was centrifuged, and the supernatant was aliquoted and stored at -70℃ until assayed. Samples were not subjected to freeze-thaw cycles before being assayed. Interleukin-6 (IL-6) concentrations were measured by an enzyme-linked immunosorbent assay human IL-6 DuoSet Kit (R&D System, Minneapolis, MN, USA). The range of the IL-6 standard curve was 7.8-600 pg/mL. The assay was carried out by strictly following the instructions provided by the manufacturer and all samples were measured in duplicate. The calculated intra- and inter-assay coefficients of variation were <10%. The maternal blood WBC count was determined using an automated hemocytometer (XE-2100; Sysmex, Tokyo, Japan). The CRP concentration was measured with a latex-enhanced turbidimetric immunoassay (Denka Seiken, Tokyo, Japan) using an automated analyzer (Toshiba 200FR; Toshiba, Tokyo, Japan).
Preterm labor was defined as the presence of regular uterine contractions with a frequency of at least two every 10 min and cervical change before 37 completed weeks of gestation that required hospitalization. The primary outcome measure was ruptured membranes in the absence of active labor occurring within 48 hr of amniocentesis. Additional analysis of the rupture of membranes after arrest of preterm labor occurring up to 7 days of amniocentesis was performed. Rupture of membranes was diagnosed by a sterile speculum examination confirming pooling of AF in the vagina and a positive nitrazine test. Intra-amniotic infection/inflammation was defined as a positive AF culture for microorganisms and/or the presence of high IL-6 levels (>2.6 ng/mL) in AF, as previously reported (4).
All patients with preterm labor and intact membranes who were admitted underwent sterile speculum examination to rule out rupture of membranes. The patients were hydrated and, if uterine contractions persisted, they were started on a regimen of intravenous tocolysis with ritodrine or magnesium sulfate after amniocentesis. Corticosteroids were administered between 24 and 34 weeks. Decisions regarding the use and type of prophylactic antibiotics were left to the discretion of the attending obstetrician. At our institution, prophylactic antibiotics are generally recommended for women with preterm labor and any of the following conditions: clinically suspected (AF WBC count ≥50 cells/µL) (13, 14) or diagnosed intra-amniotic infection, cervical dilatation with the exposure of amniotic membranes to the vagina, and the development of clinical signs of chorioamnionitis. Ampicillin was the main antibiotic used, supplemented in many cases by erythromycin or azithromycin. Tocolytic therapy, corticosteroids, and antibiotics were started after amniocentesis. Women who subsequently had PPROM after amniocentesis were managed according to a standard protocol to manage PPROM that included prophylactic antibiotics, as previously reported (15). The use of antibiotics was defined as the administration of antimicrobial agents after amniocentesis but before subsequent rupture of membranes.
The Mann-Whitney U-test was used for comparison of continuous variables owing to their non-normal distribution. Comparisons of proportions were performed with a chi-square-test or Fisher's exact test. Shapiro-Wilk and Kolmogorov-Smirnov tests were used to test for normal distribution of the data. Overall logistic regression analysis was used to investigate the independent association between the intra-amniotic infection/inflammation and the ruptured membranes occurring up to 48 hr of amniocentesis and to calculate an adjusted odds ratio. We also check for collinearity by means of the correlation matrix, using the Spearman rank correlation coefficient between independent variables. We chose a conservative strategy, with r ≥ 0.4 in at least one correlation as the criterion for multicollinearity. Next, to construct a model for risk scoring that is predictive of ruptured membranes within 48 hr of the procedure before the performance of amniocentesis, potential predictors prior to amniocentesis for ruptured membranes within 48 hr of the procedure (defined as P < 0.10) were then subjected to a stepwise forward logistic regression analysis. In this analysis, parameters (i.e., AF WBC count, AF IL-6, and AF culture results) that were associated with the risk of ruptured membranes within 48 hr of the procedure, but were unavailable at the time of a predictive risk assessment (i.e., before the start of the procedure), were omitted from the model. In this predictive model, all continuous variables were transformed into dichotomous variables because the assumption of linearity in the logic was not verified, especially where gestational age, maternal age and maternal blood WBC count were concerned. Receiver operating characteristic (ROC) curves were used to identify the best cut-off values for dichotomization of variables. All reported P values are two-sided, and P values < 0.05 were considered statistically significant. SPSS 15.0.1 for Windows (SPSS Inc, Chicago, IL, USA) was used for statistical analyses.
During the study period, 283 consecutive women with preterm labor who fulfilled the inclusion criteria were recruited for this study. Of the 283 women, AF was not available in 7 women for IL-6 determinations (all seven patients with a negative AF culture), one patient with a positive AF culture experienced an intrauterine fetal death 6 hr after amniocentesis and had an indicated preterm delivery, and 38 patients continued to have contractions and delivered their babies spontaneously within 48 hr of amniocentesis (34 patients with a negative AF culture and 4 patients with a positive AF culture). These women were excluded from the study, leaving 237 women whose contractions ceased after treatment for preterm labor, and who were judged as being suitable to evaluate the relationship between ruptured membranes occurring within 48 hr of amniocentesis and covariates. The mean (standard deviation) gestational age at the time of amniocentesis was 30.0 (3.6) weeks, with a range from 20 + 0 to 34 + 6 weeks. Of the 237 women, PPROM subsequently developed in 10 (4.2%) women within 48 hr of amniocentesis and they all spontaneously delivered preterm. Of the remaining 227 women, 6 (2.6%) women presented with ruptured membranes between 48 hr and 7 days of amniocentesis; all also spontaneously delivered preterm. None of the 46 women excluded from the study experienced fluid leakage within 48 hr of amniocentesis and, among these women, the rate of a positive AF culture was 10.9% (5/46).
The prevalence of a positive AF culture was 7.2% (17/237). The microorganisms isolated from the amniotic cavity included Ureaplasma urealyticum (n=17) and Mycoplasma hominis (n=9), with both found in nine cases. The overall rate of intra-amniotic inflammation was 20.3% (48/237). Intra-amniotic inflammation was present in all cases with a positive AF culture and intra-amniotic inflammation with a negative AF culture for microorganisms was found in 14.1% (31/220) of those with a negative AF culture.
Table 1 shows the demographic and clinical characteristics of the women according to the presence or absence of ruptured membranes occurring within 48 hr of amniocentesis. The women with rupture within 48 hr of amniocentesis had a significantly lower mean gestational age at the time of amniocentesis and higher mean serum CRP level, AF WBC count, and AF IL-6 level, and a higher rate of intra-amniotic infection/inflammation than did those without rupture and those with rupture which occurred 48 hr or more after amniocentesis. However, there were no statistically significant differences in the mean maternal age, parity, prevalence of previous spontaneous preterm delivery, and mean maternal WBC count between these two groups. A significant correlation was found among serum CRP level, maternal WBC count, AF WBC count, and AF IL-6 level (all variables; r=0.137-0.387, P < 0.05), but not to the extent to which multicollinearity is a problem in a model.
Multiple logistic regression analysis was performed to examine the relationship between the intra-amniotic infection/inflammation and ruptured membranes occurring up to 48 hr following amniocentesis after adjusting for the effect of other confounders (i.e., maternal age, gestational age, maternal serum CRP level and WBC count, AF WBC count, AF IL-6 level, use of antibiotics). Intra-amniotic infection/inflammation was significantly and independently associated with the ruptured membranes occurring within 48 hr of amniocentesis (Table 2).
As the next step, an attempt was made to develop a model with the use of logistic regression using a forward stepwise method that would be predictive of subsequent ruptured membranes prior to amniocentesis. After the exclusion of variables (i.e., AF WBC count, AF IL-6 level, and AF culture results) using amniocentesis, four variables with a significant correlation or a tendency towards an association with subsequent ruptured membranes based on univariate analysis (P < 0.10) were entered into the multivariate model (maternal age, gestational age, maternal serum CRP level, and maternal blood WBC count). The cut-off values, derived from the ROC curves and chosen to dichotomize the variables, were 15 mg/L for serum CRP, 29.0 weeks for gestational age at the time of amniocentesis, 32 yr for maternal age, and 11,800 cells/µL for maternal blood WBC count. In the predictive model, only elevated serum CRP levels (≥ 15 mg/L) was retained (odds ratio [OR], 11.982; 95% confidence interval [CI], 2.963-48.473). However, all other parameters, such as advanced maternal age and gestational age at the time of amniocentesis and elevated maternal blood WBC count, were excluded during regression analysis.
Fig. 1 depicts a ROC curve describing the values of the serum CRP level in predicting ruptured membranes within 48 hr of amniocentesis. The curve for the maternal serum CRP was above the 45° line, indicating a significant relationship with subsequent ruptured membranes (area under the curve, 0.793; standard error, 0.078; P = 0.002). The best cut-off value for serum CRP to predict subsequent ruptured membranes was 15 mg/L, with a sensitivity of 70.0%, specificity of 83.7%, positive predictive value of 15.9%, and negative predictive value of 98.5%. When the outcome measure was ruptured membranes occurring up to 7 days of amniocentesis, very similar results of univariate and multivariate analyses were obtained (data not shown).
Table 3 shows the clinical and laboratory characteristics of 16 cases presented with ruptured membranes within 7 days of amniocentesis. Fourteen cases (cases 1-12, 14, and 16 in Table 3) delivered within 7 days of amniocentesis and 71% (10/14) of these cases had elevated levels of AF IL-6 at the time of amniocentesis.
The results of our study clearly demonstrate that intra-amniotic infection/inflammation at the time of amniocentesis is significantly and independently associated with subsequent PPROM in women with preterm labor and intact membranes who have a clinically indicated amniocentesis. These observations confirm prior data showing that positive AF markers of infection are predictive of PPROM after successful arrest of preterm labor in women treated for preterm labor with intact membranes (10). Similar observations have been recently reported in the AF obtained from asymptomatic women at the time of mid-trimester genetic amniocentesis (11, 12, 16).
A body of evidence derived from microbiologic, epidemiologic and immunological studies has indicated a causal link between infection/inflammation and subsequent PPROM (11, 12, 17, 18). Infection/inflammation-related PPROM is initiated by stimulation of pro-inflammatory cytokine production, which modulates the expression of MMPs in amniochorionic cells (19, 20). Similarly, among the risk factors examined in the current study, only intra-amniotic infection/inflammation was found to be associated with membrane rupture occurring up to 48 hr after amniocentesis in the multivariable model, suggesting that intra-amniotic infection/inflammation antedated the procedure and, thus, can predispose women with preterm labor to PPROM. This finding has important medico-legal implications and may be useful in the case of litigations, because spontaneous rupture of membranes after amniocentesis in women with preterm labor may be related to a pre-existing infection/inflammation process, rather than the procedure itself. On the other hand, the present absence of elevated AF IL-6 levels (> 2.6 ng/mL) in two patients with rupture occurring up to 48 hr within amniocentesis (Table 3) may reflect the multifactorial etiology of PPROM, including the procedure itself, or may simply reflect timing of sample collection.
The model for prediction of ruptured membranes occurring up to 48 hr of the procedure was chosen to obtain information prior to amniocentesis. We found that only elevated serum CRP levels provided the optimal prediction of subsequent ruptured membranes prior to amniocentesis. In the current study, a serum CRP level ≥ 15 mg/L had 70.0% sensitivity and 83.7% specificity for subsequent ruptured membranes after amniocentesis. On the other hand, 98.5% of women with a serum CRP of < 15 mg/L did not experience AF leakage after amniocentesis, suggesting a very good negative predictive value. With a high negative predictive value, this test can identify women with preterm labor who are not at risk for the subsequent development of PPROM in the amniocentesis setting. Such information may be useful for counseling women with preterm labor regarding the risks and benefits of assessing the microbiologic status of the amniotic cavity and/or fetal lung maturity with amniocentesis.
We observed the pregnancy courses of 16 women with ruptured membranes occurring up to 7 days after amniocentesis (Table 3). These pregnancies had a worse pregnancy outcome, with all involving spontaneous preterm delivery, regardless of the presence or absence of intra-amniotic infection/inflammation. These observations were in contrast to the study of Borgida et al. (21), which showed that 5 of 11 women with ruptured membranes after mid-trimester genetic amniocentesis delivered their infants at term. However, discrepancy between these two studies is natural, given that subsequent ruptured membranes after amniocentesis in preterm labor, which commonly results from pathologic processes (22), may represent a fundamentally different entity compared with ruptured membranes after mid-trimester genetic amniocentesis.
In the present study, the rates of positive AF cultures and intra-amniotic inflammation were similar to those observed by others (2, 3). However, the prevalence of the subsequent development of PPROM up to 7 days after amniocentesis in the current study was 6.8%, which is significantly lower than the 17.4% (P < 0.001) that Guinn et al. (10) previously reported in a secondary analysis of a multicenter randomized trial. The discrepancy between these studies is not clear, but may be related to the differences between the inclusion criteria pertaining to the cervical dilatation at which women were enrolled (cervical dilatation ≤ 4 cm vs ≤ 3 cm) and the fact that, over the last 20 yr, advanced technology, including better equipment of ultrasound and needle, and improvements in amniocentesis techniques may have improved the safety of amniocentesis. When compared with our data, the data from Guinn et al. (10) had more advanced cervical dilatation at the time of enrollment. Furthermore, patients in the previous study (10) were vulnerable to subsequent development of PPROM after amniocentesis because an advanced cervical dilation is often associated with intra-amniotic infection/inflammation and the membrane stretch that weaken the membranes (3, 23).
There are several limitations in this study. First, the retrospective nature of this study introduces the issue of inherent bias. However, most data were collected prospectively prior to inclusion in the study and the primary outcome variable was reliably recorded in the electronic medical record system because PPROM was diagnosed in each hospitalized patient. Second, a small number of the ruptured membranes occurring up to 48 hr of amniocentesis were documented, prohibiting us from drawing valid conclusion from these data. Further large prospective studies are needed to confirm our findings. Third, AFs in the present study were not measured for MMPs, which were known to weaken the fetal membranes leading to rupture (19, 24). Therefore, we could not elucidate the mechanism by which intra-amniotic infection/inflammation can predispose a woman with preterm labor to develop subsequent PPROM. Fourth, our study did not include the number of needle passes per procedure and whether the needle traversed the placenta, which might result in a higher rate of ruptured membranes, as covariates because this information was not well recorded for many subjects.
In conclusion, we demonstrate that intra-amniotic infection/inflammation is an independent risk factor for subsequent ruptured membranes after clinically indicated amniocentesis in preterm labor. Prior to amniocentesis, measurement of serum CRP level can provide a risk assessment for the subsequent development of PPROM after the procedure.
Figures and Tables
Fig. 1
Receiver operating characteristic curve for maternal serum C-reactive protein (CRP) in predicting ruptured membranes within 48 hr of amniocentesis. Numbers next to solid dots represent serum CRP levels (mg/L; area under the curve, 0.793; SE, 0.078; P = 0.001).
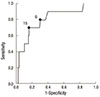
Table 1
Demographic and clinical characteristics of the study population according to the presence or absence of ruptured membranes occurring within 48 hr of amniocentesis

References
1. Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007; 25:21–39.
2. Hitti J, Hillier SL, Agnew KJ, Krohn MA, Reisner DP, Eschenbach DA. Vaginal indicators of amniotic fluid infection in preterm labor. Obstet Gynecol. 2001; 97:211–219.
3. Palacio M, Cobo T, Bosch J, Filella X, Navarro-Sastre A, Ribes A, Gratacós E. Cervical length and gestational age at admission as predictors of intra-amniotic inflammation in preterm labor with intact membranes. Ultrasound Obstet Gynecol. 2009; 34:441–447.
4. Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001; 185:1130–1136.
5. Holst RM, Jacobsson B, Hagberg H, Wennerholm UB. Cervical length in women in preterm labor with intact membranes: relationship to intra-amniotic inflammation/microbial invasion, cervical inflammation and preterm delivery. Ultrasound Obstet Gynecol. 2006; 28:768–774.
6. Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, Nien JK, Berry SM, Bujold E, Camacho N, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006; 34:13–19.
7. Morency AM, Rallu F, Laferrière C, Bujoldg E. Eradication of intra-amniotic Streptococcus mutans in a woman with a short cervix. J Obstet Gynaecol Can. 2006; 28:898–902.
8. Mazor M, Chaim W, Hershkowitz R, Wiznitzer A. Eradication of Viridans streptococci from the amniotic cavity with transplacental antibiotic treatment. Arch Gynecol Obstet. 1994; 255:147–151.
9. Gordon MC, Narula K, O'Shaughnessy R, Barth WH Jr. Complications of third-trimester amniocentesis using continuous ultrasound guidance. Obstet Gynecol. 2002; 99:255–259.
10. Guinn DA, Goldenberg RL, Hauth JC, Andrews WW, Thom E, Romero R. Risk factors for the development of preterm premature rupture of the membranes after arrest of preterm labor. Am J Obstet Gynecol. 1995; 173:1310–1315.
11. Biggio JR Jr, Ramsey PS, Cliver SP, Lyon MD, Goldenberg RL, Wenstrom KD. Midtrimester amniotic fluid matrix metalloproteinase-8 (MMP-8) levels above the 90th percentile are a marker for subsequent preterm premature rupture of membranes. Am J Obstet Gynecol. 2005; 192:109–113.
12. Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, Kalish RB, Witkin SS. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004; 191:1382–1386.
13. Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, Ramirez M, Fidel PL, Sorokin Y, Cotton D, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993; 169:805–816.
14. Yoon BH, Yang SH, Jun JK, Park KH, Kim CJ, Romero R. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: a comparison with amniotic fluid white blood cell count. Obstet Gynecol. 1996; 87:231–237.
15. ACOG Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 80: premature rupture of membranes: clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2007; 109:1007–1019.
16. Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, DuBard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol. 1998; 178:546–550.
17. Ekwo EE, Gosselink CA, Woolson R, Moawad A. Risks for premature rupture of amniotic membranes. Int J Epidemiol. 1993; 22:495–503.
18. Alger LS, Lovchik JC, Hebel JR, Blackmon LR, Crenshaw MC. The association of Chlamydia trachomatis, Neisseria gonorrhoeae, and group B streptococci with preterm rupture of the membranes and pregnancy outcome. Am J Obstet Gynecol. 1988; 159:397–404.
19. Vadillo-Ortega F, Estrada-Gutiérrez G. Role of matrix metalloproteinases in preterm labour. BJOG. 2005; 112:19–22.
20. Vadillo-Ortega F, Sadowsky DW, Haluska GJ, Hernandez-Guerrero C, Guevara-Silva R, Gravett MG, Novy MJ. Identification of matrix metalloproteinase-9 in amniotic fluid and amniochorion in spontaneous labor and after experimental intrauterine infection or interleukin-1 beta infusion in pregnant rhesus monkeys. Am J Obstet Gynecol. 2002; 186:128–138.
21. Borgida AF, Mills AA, Feldman DM, Rodis JF, Egan JF. Outcome of pregnancies complicated by ruptured membranes after genetic amniocentesis. Am J Obstet Gynecol. 2000; 183:937–939.
22. Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994; 734:414–429.
23. Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol. 1993; 30:167–183.
24. Vadillo-Ortega F, Hernandez A, Gonzalez-Avila G, Bermejo L, Iwata K, Strauss JF 3rd. Increased matrix metalloproteinase activity and reduced tissue inhibitor of metalloproteinases-1 levels in amniotic fluids from pregnancies complicated by premature rupture of membranes. Am J Obstet Gynecol. 1996; 174:1371–1376.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download