Abstract
In 2009, the first outbreak of hand, foot and mouth disease (HFMD) or herpangina (HP) caused by enterovirus 71 occurred in the Republic of Korea. This study inquired into risk factors associated with complications of HFMD or HP. A retrospective medical records review was conducted on HFMD or HP patients for whom etiologic viruses had been verified in 2009. One hundred sixty-eight patients were examined for this investigation. Eighty patients were without complications while 88 were accompanied by complications, and 2 had expired. Enterovirus 71 subgenotype C4a was the most prevalent in number with 67 cases (54.9%). In the univariate analysis, the disease patterns of HFMD rather than HP, fever longer than 4 days, peak body temperature over 39℃, vomiting, headache, neurologic signs, serum glucose over 100 mg/dL, and having an enterovirus 71 as a causative virus were significant risk factors of the complications. After multiple logistic analysis, headache (Odds ratio [OR], 10.75; P < 0.001) and neurologic signs (OR, 42.76; P < 0.001) were found to be the most significant factors. Early detection and proper management of patients with aforementioned risk factors would be necessary in order to attain a better clinical outcome.
Hand, foot and mouth disease (HFMD) is a common acute viral illness with fever, oral ulcers, and vesicular rashes on the hands, feet and buttock as characteristics features. HFMD occurs most frequently by coxsackievirus A16, and is caused by various enteroviruses including enterovirus 71. Herpangina (HP) has characteristics of having fever and oral ulcers without skin rash, and is developed by various enteroviruses likewise HFMD. HFMD or HP is generally known as a self-limiting disease that shows a mild clinical course. However, enterovirus 71-induced HFMD or HP may often show a severe clinical course, accompanied by neurologic complications, and may lead to death (1).
Ever since enterovirus 71 was first recognized in California in 1969, it has been epidemic everywhere in the world (2, 3). Particularly, in the late 1990s, enterovirus 71-caused HFMD and HP largely developed and continuously led to death due to their complications in the Asian countries of the Western Pacific such as Malaysia (4), Taiwan (5), Japan (6), Singapore (7), and China (8). The Republic of Korea (ROK) is located geographically adjacent to these countries. Ever since enterovirus 71-caused sporadic cases of the year 2000 developed in the ROK (9), enterovirus 71-induced epidemic or death did not occur until 2009 when another epidemic and associated death by enterovirus 71-caused HFMD surfaced (10). Thus, some cases, especially enterovirus 71-caused HFMD or HP patients, may show a clinical course of rapid exacerbation accompanied by complications. Accordingly, understanding of risk factors accompanied by complications and their management are imperative.
Two study results were already reported on the ROK's epidemic in 2009 (10, 11). Nevertheless, these studies utilized merely the data entered into the Nationwide Enterovirus Sentinel Surveillance System and did not include detailed medical records review on each case. Thus, this study sought risk factors associated with complications after examined the clinical features and laboratory findings though the medical records of patients admitted to the hospital with HFMD or HP confirmed for etiologic viruses, which were endemic in the ROK in 2009.
The Enterovirus Sentinel Surveillance System in the ROK consists of 8 primary hospitals, 12 secondary hospitals and 40 tertiary hospitals. Specimens from patients suspected of enteroviruses infection are sent to the Division of Hepatitis and Enteric Viruses, Korea Center for Disease Control and Prevention (KCDC). Patients' clinical data are entered into the web-based system. Using specimens from stool, throat swab and cerebrospinal fluid (CSF), cell culture, PCR and genetic sequencing are carried out with respect to enteroviruses at the KCDC.
Two thousand four-hundred twenty-seven cases suspected of enteroviruses infection were registered into the Enterovirus Sentinel Surveillance System from January through December, 2009. Among them, 519 cases were HFMD or HP and enteroviruses were detected in 321 patients from 24 hospitals. This study excluded 132 outpatients and requested medical record review on 189 patients who were admitted and treated at each hospital. Among them, a total of 168 patients were able to undergo a medical record review (Fig. 1). Clinical data were collected by each hospital in structured manner for the period from April through August, 2010.
One to 3 specimens among throat swab, endotracheal aspirates, rectal swab, stool or CSF were collected depending on each patient. Enteroviruses genome detection was attempted by real-time reverse transcription-PCR (RT-PCR) by using TaqMan technology (Applied Biosystems, Foster City, CA, USA). A causative organism was determined in cases of detecting one or more viruses among them.
Briefly, viral RNAs were extracted by using the magnetic bead-based viral nucleic acid purification protocol described by Boom et al. (12). Subsequently, 1-step real-time RT-PCR was performed by using a dual-labeled fluorogenic enteroviruses-specific probe and primers designed on the basis of previous data (13). For genotyping, seminested RT-PCR was used to amplify part of the viral protein 1 (VP1) gene of enteroviruses, based on the KCDC protocol for detection of pan-enteroviruses, and sequencing analysis for VP1 amplicon was performed by using the automatic sequencer and the DNAstar software package (14).
HFMD was defined as having vesiculo-papular rashes over the hands, soles, or buttocks with mouth ulcers while HP was defined as having oral ulcers without any skin rash. Cases with development of complications were categorized as the cases accompanied by aseptic meningitis, encephalitis, encephalomyelitis, and polio-like syndrome (PLS). Aseptic meningitis was defined as having clinical signs or symptoms of meningitis with pleocytosis (leukocyte > 5 cells/µL) with negative bacterial culture in the CSF obtained from a lumbar puncture. Encephalitis was defined as having CSF pleocytosis with a change in consciousness. PLS was defined as having acute weakness in the extremities with a drop in muscle power or deep tendon reflex. Encephalomyelitis was defined as having encephalitis and PLS simultaneously.
Data were collected for demographic information, past medical history, contact history of enteroviruses associated diseases, vital signs (the presence of fever, peak body temperature, etc.), symptoms and signs at presentation during the whole course of disease. Collected also were laboratory data including white blood cell and platelet count, hemoglobin, C-reactive protein (CRP), erythrocyte sedimentation rate, blood glucose and cell counts, glucose and protein in CSF, and isolated virus and subtype.
We analyzed the data using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). We analyzed fever duration, peak fever, clinical symptom (vomiting, diarrhea, poor oral intake, headache), neurologic sign (jerking, gaze palsy, tremor, dysarthria, ataxia, gait disturbance, upper or lower extremity weakness, insomnia, seizure, lethargy, coma), laboratory test (white blood cell count, erythrocyte sedimentation rate, CRP, glucose) and isolated virus type as a risk factor. Normally distributed data were compared using Student's t test; data that were not normally distributed were compared by the Mann-Whitney U test. Categorical data were tested using the chi-square test or Fisher's exact test. A multiple logistic regression analysis was used to examine the multivariate-adjusted odds ratios for risk factors that were significant in univariate analysis. A P value less than 0.05 was considered statistically significant.
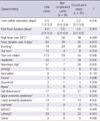
Among 189 patients from 24 hospitals subject to investigation, 168 patients (88.9%) from 21 hospitals (87.5%) underwent a medical record review. Among these subjects, 80 patients (47.6%) were not accompanied by complications while 88 patients (52.4%) had complications. Of those 88 patients that developed complications, 56 patients (33.3%) had aseptic meningitis, 18 patients (10.7%) had encephalitis or encephalomyelitis, and 14 patients (8.3%) had PLS. Of those 18 subjects with encephalitis or encephalomyelitis, 4 patients (25%) were accompanied by cardiopulmonary complications and 2 of these 4 patients expired (Table 1).
Of those 168 patients who were investigated, 103 patients (68.3%) were males and 65 patients (38.7%) were females with the ratio of 1.58:1. The overall median age was 28.0 months (with a range 16.5-49.5). With respect to the age at which the disease occurs, the peak incidence was shown just before and after 12 months. Ninety two point nine percent of these patients were less than 5 yr old (152/168) (Fig. 2). There was no statistically significant difference in the male-female ratio between the group with complications and the group without complications, but the group with complications was significantly older (P = 0.041) and heavier (P = 0.002), and had a longer hospital stay (P < 0.001) (Table 2).
The patients showed distinctive seasonal prevalence. A death occurred in early May and the number of patients rapidly increased thereafter showing the peak incidence in near summer between May and July (Fig. 3).
Patients were admitted to the hospital with fever that lasted for the median period of 2 days prior to admission. Fever continued for the median period of 4 days. Of those 168 subjects examined, 133 patients (79.2%) had HFMD while 35 patients (20.8%) had HP. The group with complications had more incidences of HFMD than HP (P = 0.002), had a longer period of fever (P = 0.001) and a higher fever over 39℃ (P = 0.028). This group more frequently showed headache (P < 0.001), vomiting (P < 0.001) and one or more neurologic signs/symptoms (P < 0.001). In the group with complications, ataxia, gait disturbance, lower extremity weakness, seizure, lethargy and coma, among above neurologic signs and symptoms, were shown statistically more often (Table 3).
There was no statistical difference in ratio of patients with leukocytosis between the group with complications and without complication (P = 0.465). The mean CRP of the group with complications (1.09 ± 2.05 mg/dL) was lower than that of the group without complications (1.47 ± 1.81 mg/dL) (P = 0.022). The mean serum glucose of the group with complications was 110.3 ± 30.8 mg/dL while that of the group without complications was 98.3 ± 26.4 mg/dL, showing a higher level for the former (P = 0.003) (Table 4).
Throat swabs from 92 patients were obtained for virus isolation and 51 (55.4%) were positive for enteroviruses. Of those 154 patients who had a rectal swab or stool sample for virus isolation, 146 (94.8%) were positive. However, 40 patients had a CSF sample for virus isolation and only 3 patients (7.5%) were positive (Fig. 4).
Enterovirus 71 was isolated in 122 patients (72.6%), showing the largest number of cases. Other isolated viruses detected were coxsackievirus A2, A5, A6, A12, A16, coxsackievirus B1, echovirus 9 and untypable enterovirus. Among cases with enterovirus 71 isolation, 67 cases (54.9%) belonged to the subgenogroup C4a. This was shown to be 97% of homologous to enterovirus 71 subgenogroup that was endemic in China, 2008.
The group with complications and the group without complications were compared by univariate analysis. It was found that significant risk factors for complications included a rash form of HFMD, fever longer than 4 days, peak body temperature over 39℃, vomiting, headache, neurologic sign (such as jerking, gaze palsy, tremor, dysarthria, ataxia, gait disturbance, extremity weakness, insomnia, seizure, lethargy, coma), glucose over than 100 mg/dL and enterovirus 71 as a causative virus. After multiple logistic analysis, headache (Odds ratio [OR], 10.75; P < 0.001) and neurologic sign (OR, 42.76; P < 0.001) were the most significant factors (Table 5).
In the late 1990s, the large outbreaks of HFMD or HP caused by enterovirus 71 continuously occurred in the neighbor countries of the ROK and many deaths had occurred (4-8). Thus, a large outbreak of HFMD or HP was also a matter of concern in the ROK. According to the Enterovirus Sentinel Surveillance System of the ROK, there was no HFMD or HP caused by enterovirus 71 up until 2008, but the outbreaks of HFMD or HP caused by enterovirus 71 and deaths occurred from the year 2009. Accordingly, this study attempted to understand more accurately the epidemic aspect of large outbreaks of HFMD or HP that occurred in the ROK in 2009, and to analyze the risk factors associated with complications.
In this study, the ratio of males and females of HFMD or HP incidences was 1.58:1 while 93.8% of all patients were under the age of five years. The largest number of patients was especially around the age of one year. Deaths occurred at around the age of one year, despite the fact that only two had expired. This result concurred with that of other studies (15-18).
As the patients are younger, there are higher possibility of complications and death from HFMD or HP. Nevertheless, neurologic signs are often subtle and easily overlooked (19, 20). Thus, in times of HFMD or HP epidemic, careful observations with respect to occurrences of complications of patients are necessary in their early childhood. The HFMD or HP epidemic in the ROK was manifested largely in the period from May through August, 2009. It almost did not occur after October that year, which was similar to what was seen in other neighbor countries (15, 20). However, owing to the possibility of a continuous epidemic regardless of any particular season, it is necessary to put together a well-built sentinel surveillance system and to establish an early warning system for the national public in times of an epidemic.
Complications of HFMD or HP disclosed the spectrum of disease severity. Complications began largely in the form of aseptic meningitis. Then, they progressed to the state of meningoencephalitis that manifested a change of consciousness. However, some patients did not show any change of consciousness, instead of complications progressed to PLS. Well-known already was the report that enterovirus 71 caused complications in the form of not only meningoencephalitis but also PLS (21). Enterovirus 71 virus is on the rise as the most important causative virus for the PLS in the Western Pacific region where polio viruses had been eradicated (22).
It was reported that enterovirus 71 that often brought about complications would have a tissue tropism in the brain stem, cerebellum and spinal cord anterior horn cell (23). Clinical manifestations may change depending on the body tissues where these viruses first act upon. The possibility of complications progression is high in cases of change of consciousness such as irritability or lethargy, as well as manifestations of neurologic symptoms, such as ataxia, tremor, lower extremity weakness, inability to walk and decreased deep tendon reflex. Thus, careful observations and effective measures for these symptoms would be necessary.
In this study, hyperglycemia and leukocytosis at admission were not statistically significant risk factors of complications. These findings were not present on admission of the expired patients in this study, only manifested after witnessing declining consciousness and the worsening patient's condition. The results of this study led us to believe that clinicians would not be able to predict looming complications with mere general laboratory findings. However, the finding of hyperglycemia or leukocytosis in patients with HFMD or HP may already reflect the considerable progression of complications.
Chen et al. (22) reported, akin to the results of this study, that hyperglycemia or leukocytosis were signs after the progression of the disease itself, rather than risk factors of complications. However, there were reports in which hyperglycemia and leukocytosis were investigated as the risk factors for fatal enterovirus 71 infections (19, 25).
In this study, the mean CRP was significantly lower in the complications group. It might reflect that inflammatory responses were low in the complications group. Owing to weak inflammatory responses toward viruses in patients with HFMD or HP accompanied by complications, viral proliferation would not effectively be suppressed. As a result, a severe clinical progression might ensue. Additional study is necessary for this pathophysiologic mechanism.
The viral diagnostic sensitivity was the highest in the stool specimens in this study. However, according to Ooi et al. (26), the diagnostic sensitivity for enterovirus 71 was the highest in the throat swab specimens. It is advisable for clinicians to take two or more specimens in order to increase diagnostic sensitivity. This study showed that the enterovirus 71 subgenogroup epidemic in the ROK, 2009, was enterovirus 71 C4a. This was 97% homologous to enterovirus 71 C4a, which had been prevalent in China, 2008 (8). Nevertheless, enterovirus 71 was not an epidemic in the ROK in 2008. Thus, the enterovirus 71 epidemic in China might have been introduced to the ROK in 2009. A report claimed that a gradual increase in recent international travel often introduce epidemic viruses from one country to neighbor countries (27). Thus, it is necessary to monitor not only the state of the domestic enterovirus 71 epidemic but also of other countries.
The HFMD or HP patients with neurologic complications could progress to death within a few days after development of fever. However, at primary hospital, diagnosis and treatment while conducting tests searching for causative viruses would not be possible in reality. Therefore, it is of utmost importance to find risk factors associated with development of complications based on clinical symptoms. In this study, rash patterns of HFMD rather than HP, fever longer than 4 days, peak body temperature over 39℃, vomiting, headache and neurologic signs were associated with complications. Also, neurologic signs included ataxia, gait disturbance, lower extremity weakness, seizure, lethargy and coma. Ooi et al. (24) reported that fever longer than 3 days, peak temperature over 38.5℃ and history of lethargy were identified as independent risk factors for a neurological involvement. Chang et al. (19) reported that fever for longer than 3 days, peak body temperature over 39℃, headache, lethargy, vomiting, and seizure were associated with a central nervous system involvement with an enterovirus 71 infection. Putting these various reports together up to now, higher fever, longer fever duration, as well as meningeal irritation or other neurologic signs, are common risk factors of central nervous system complications. In times of HFMD or HP epidemic, early detection and timely proper management of patients with such risk factors are essential.
Until now, there is no vaccine or antiviral agent effective against enteroviruses-caused HFMD or HP. However, there was a report, although additional verifications are required, that timely administration of intravenous immunoglobulin (IVIG) for severe enterovirus 71 patients had reduced acute mortality (28-30). Thus, attempts of close monitoring and timely IVIG infusion would be necessary for patients with the above mentioned risk factors.
Our study has some limitations due to its retrospective nature. Firstly, we did not investigate potentially important epidemiologic factors likewise number of children and adults in a family, history of HFMD or HP before admission, intrafamilial or outside contact with HFMD or HP, enrollment in a kindergarten or child care center. Secondly, each patient's specimens were not collected at the same time from disease onset. Thirdly, we investigated only hospitalized patient, therefore our result may be not applicable to primary care setting.
In conclusion, in cases of having rash patterns of HFMD rather than HP, fever longer than 4 days, peak body temperature over 39℃, vomiting, headache, neurologic signs, serum glucose over 100 mg/dL and enterovirus 71 as a causative virus might be indicative of a grave prognosis in times of HFMD or HP epidemic. Therefore, early recognition and especially meticulous management in patients with these risk factors are requisite.
Figures and Tables
Fig. 1
The flow chart shows how we selected the study population in this study. EV, enterovirus; HFMD, hand, foot and mouth disease; HP, herpangina; KCDC, Korea Centers for Disease Control and Prevention.

Fig. 2
Number of hand, foot and mouth disease or herpangina in the Republic of Korea in 2009, by age group. mo, months; yr, years.

Fig. 3
Distribution of hand, foot and mouth disease or herpangina in the Republic of Korea in 2009, by date. The arrows indicate the dates of onset of the two fatal cases.

Fig. 4
Origins and numbers (percentage) of enterovirus positive samples on RT-PCR in the Republic of Korea in 2009. CSF, cerebrospinal fluid.

Table 1
Classification of various clinical syndrome of virus confirmed hand, foot and mouth disease or herpangina

Table 2
Demographic characteristics of each complicated versus non-complicated group of hand, foot and mouth disease or herpangina in the Republic of Korea, by age group on 2009

Table 3
Clinical manifestations of each complicated versus non-complicated group of hand, foot and mouth disease or herpangina in the Republic of Korea in 2009, by age group
ACKNOWLEDGMENTS
Members of the Enteroviruses Complications Working Group
The following investigators and institutions participated in the Enteroviruses Complications Working Group other than those listed authors of this paper: Byung Min Choi, Department of Pediatrics, College of Medicine, Korea University, Seoul; Eun Hwa Choi, Department of Pediatrics, Seoul National University College of Medicine, Seoul; Jae Yoon Kim, Department of Pediatrics, National Medical Center, Seoul; Jung Yeun Hong, Department of Pediatrics, School of Medicine, Cheju National University, Cheju; Sung Hee Oh, Department of Pediatrics, Hanyang University School of Medicine, Seoul; Sung Ho Cha, Department of Pediatrics, College of Medicine, Kyunghee University, Seoul; Yae-Jean Kim, Department of Pediatrics, Sungkyunkwan University School of Medicine, Seoul, Korea. The authors have no conflicts of interest to disclose.
References
1. Lum LC, Wong KT, Lam SK, Chua KB, Goh AY, Lim WL, Ong BB, Paul G, AbuBakar S, Lambert M. Fatal enterovirus 71 encephalomyelitis. J Pediatr. 1998. 133:795–798.
2. Schmidt NJ, Lennette EH, Ho HH. An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis. 1974. 129:304–309.
3. Landry ML, Fonseca SN, Cohen S, Bogue CW. Fatal enterovirus type 71 infection: rapid detection and diagnostic pitfalls. Pediatr Infect Dis J. 1995. 14:1095–1100.
4. Shekhar K, Lye MS, Norlijah O, Ong F, Looi LM, Khuzaiah R, Marzuki I, Hussein I, Wong SL, Mohan J, et al. Deaths in children during an outbreak of hand, foot and mouth disease in Peninsular Malaysia: clinical and pathological characteristics. Med J Malaysia. 2005. 60:297–304.
5. Ho M, Chen ER, Hsu KH, Twu SJ, Chen KT, Tsai SF, Wang JR, Shih SR. Taiwan Enterovirus Epidemic Working Group. An epidemic of enterovirus 71 infection in Taiwan. N Engl J Med. 1999. 341:929–935.
6. Fujimoto T, Chikahira M, Yoshida S, Ebira H, Hasegawa A, Totsuka A, Nishio O. Outbreak of central nervous system disease associated with hand, foot, and mouth disease in Japan during the summer of 2000: detection and molecular epidemiology of enterovirus 71. Microbiol Immunol. 2002. 46:621–627.
7. Shah VA, Chong CY, Chan KP, Ng W, Ling AE. Clinical characteristics of an outbreak of hand, foot and mouth disease in Singapore. Ann Acad Med Singapore. 2003. 32:381–387.
8. Zhang Y, Tan XJ, Wang HY, Yan DM, Zhu SL, Wang DY, Ji F, Wang XJ, Gao YJ, Chen L, et al. An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. J Clin Virol. 2009. 44:262–267.
9. Jee YM, Cheon DS, Kim K, Cho JH, Chung YS, Lee J, Lee SH, Park KS, Lee JH, Kim EC, et al. Genetic analysis of the VP1 region of human enterovirus 71 strains isolated in Korea during 2000. Arch Virol. 2003. 148:1735–1746.
10. Ryu W, Kang B, Hong J, Hwang S, Kim A, Kim J, Cheon DS. Enterovirus 71 infection with central nervous system involvement, South Korea. Emerg Infect Dis. 2010. 16:1764–1766.
11. Ryu WS, Kang B, Hong J, Hwang S, Kim J, Cheon DS. Clinical and etiological characteristics of enterovirus 71-related diseases during a recent 2-year period in Korea. J Clin Microbiol. 2010. 48:2490–2494.
12. Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990. 28:495–503.
13. Tan EL, Yong LL, Quak SH, Yeo WC, Chow VT, Poh CL. Rapid detection of enterovirus 71 by real-time TaqMan RT-PCR. J Clin Virol. 2008. 42:203–206.
14. Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol. 2006. 44:2698–2704.
15. Tseng FC, Huang HC, Chi CY, Lin TL, Liu CC, Jian JW, Hsu LC, Wu HS, Yang JY, Chang YW, et al. Epidemiological survey of enterovirus infections occurring in Taiwan between 2000 and 2005: analysis of sentinel physician surveillance data. J Med Virol. 2007. 79:1850–1860.
16. Kung SH, Wang SF, Huang CW, Hsu CC, Liu HF, Yang JY. Genetic and antigenic analyses of enterovirus 71 isolates in Taiwan during 1998-2005. Clin Microbiol Infect. 2007. 13:782–787.
17. Ortner B, Huang CW, Schmid D, Mutz I, Wewalka G, Allerberger F, Yang JY, Huemer HP. Epidemiology of enterovirus types causing neurological disease in Austria 1999-2007: detection of clusters of echovirus 30 and enterovirus 71 and analysis of prevalent genotypes. J Med Virol. 2009. 81:317–324.
18. Ma E, Lam T, Chan KC, Wong C, Chuang SK. Changing epidemiology of hand, foot, and mouth disease in Hong Kong, 2001-2009. Jpn J Infect Dis. 2010. 63:422–426.
19. Chang LY, Lin TY, Hsu KH, Huang YC, Lin KL, Hsueh C, Shih SR, Ning HC, Hwang MS, Wang HS, et al. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet. 1999. 354:1682–1686.
20. Podin Y, Gias EL, Ong F, Leong YW, Yee SF, Yusof MA, Perera D, Teo B, Wee TY, Yao SC, et al. Sentinel surveillance for human enterovirus 71 in Sarawak, Malaysia: lessons from the first 7 years. BMC Public Health. 2006. 6:180.
21. Huang CC, Liu CC, Chang YC, Chen CY, Wang ST, Yeh TF. Neurologic complications in children with enterovirus 71 infection. N Engl J Med. 1999. 341:936–942.
22. Chen CY, Chang YC, Huang CC, Lui CC, Lee KW, Huang SC. Acute flaccid paralysis in infants and young children with enterovirus 71 infection: MR imaging findings and clinical correlates. AJNR Am J Neuroradiol. 2001. 22:200–205.
23. Hsueh C, Jung SM, Shih SR, Kuo TT, Shieh WJ, Zaki S, Lin TY, Chang LY, Ning HC, Yen DC. Acute encephalomyelitis during an outbreak of enterovirus type 71 infection in Taiwan: report of an autopsy case with pathologic, immunofluorescence, and molecular studies. Mod Pathol. 2000. 13:1200–1205.
24. Ooi MH, Wong SC, Mohan A, Podin Y, Perera D, Clear D, del Sel S, Chieng CH, Tio PH, Cardosa MJ, et al. Identification and validation of clinical predictors for the risk of neurological involvement in children with hand, foot, and mouth disease in Sarawak. BMC Infect Dis. 2009. 9:3.
25. Yang TT, Huang LM, Lu CY, Kao CL, Lee WT, Lee PI, Chen CM, Huang FY, Lee CY, Chang LY. Clinical features and factors of unfavorable outcomes for non-polio enterovirus infection of the central nervous system in northern Taiwan, 1994-2003. J Microbiol Immunol Infect. 2005. 38:417–424.
26. Ooi MH, Solomon T, Podin Y, Mohan A, Akin W, Yusuf MA, del Sel S, Kontol KM, Lai BF, Clear D, et al. Evaluation of different clinical sample types in diagnosis of human enterovirus 71-associated hand-foot-and-mouth disease. J Clin Microbiol. 2007. 45:1858–1866.
27. Mizuta K, Abiko C, Murata T, Matsuzaki Y, Itagaki T, Sanjoh K, Sakamoto M, Hongo S, Murayama S, Hayasaka K. Frequent importation of enterovirus 71 from surrounding countries into the local community of Yamagata, Japan, between 1998 and 2003. J Clin Microbiol. 2005. 43:6171–6175.
28. Wang J, Yao C, Yeh C, Huang CC, Wang SM, Liu CC, Wu JM. Critical management in patients with severe enterovirus 71 infection. Pediatr Int. 2006. 48:250–256.
29. Chang LY, Hsia SH, Wu CT, Huang YC, Lin KL, Fang TY, Lin TY. Outcome of enterovirus 71 infections with or without stage-based management: 1998 to 2002. Pediatr Infect Dis J. 2004. 23:327–332.
30. Wang SM, Lei HY, Huang MC, Su LY, Lin HC, Yu CK, Wang JL, Liu CC. Modulation of cytokine production by intravenous immunoglobulin in patients with enterovirus 71-associated brainstem encephalitis. J Clin Virol. 2006. 37:47–52.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download