Abstract
Turner syndrome is well known to be associated with significant cardiovascular abnormalities. This paper studied the incidence of cardiovascular abnormalities in asymptomatic adolescent patients with Turner syndrome using multidetector computed tomography (MDCT) instead of echocardiography. Twenty subjects diagnosed with Turner syndrome who had no cardiac symptoms were included. Blood pressure and electrocardiography (ECG) was checked. Cardiovascular abnormalities were checked by MDCT. According to the ECG results, 11 had a prolonged QTc interval, 5 had a posterior fascicular block, 3 had a ventricular conduction disorder. MDCT revealed vascular abnormalities in 13 patients (65%). Three patients had an aberrant right subclavian artery, 2 had dilatation of left subclavian artery, and others had an aortic root dilatation, aortic diverticulum, and abnormal left vertebral artery. As for venous abnormalities, 3 patients had partial anomalous pulmonary venous return and 2 had a persistent left superior vena cava. This study found cardiovascular abnormalities in 65% of asymptomatic Turner syndrome patients using MDCT. Even though, there are no cardiac symptoms in Turner syndrome patients, a complete evaluation of the heart with echocardiography or MDCT at transition period to adults must be performed.
Turner syndrome is a disease caused by a total or partial loss of an X chromosome in women, showing a frequency of 1 per 2,500-4,000 live-born females (1, 2). It is a well-known fact that the frequency of characteristics, such as short stature, gonadal dysgenesis, webbed neck, or cubitus valgus, and congenital cardiovascular anomalies, such as aortic coarctation and bicuspid aortic valve, is high in Turner syndrome (3-5). The frequency of cardiovascular anomalies in Turner syndrome is reported to be 23 to 45% (3, 6).
These cardiovascular abnormalities can cause complications, such as aortic dissection or aortic dilatation (7), and these complications are also related to the mortality rate in Turner syndrome. Additionally, reports have also revealed arterial and venous abnormalities other than aortic disorders, such as an aberrant right subclavian artery, persistent left superior vena cava (8), and partial anomalous pulmonary venous return (4, 6, 9-12). To evaluate the cardiovascular abnormalities in Turner syndrome, echocardiography is widely available, but it is limited by the chest wall anomalies in Turner syndrome, which can lead to a suboptimal imaging. Recently, a reassessment of the cardiovascular system around the time of transitioning from pediatric to adult care in Turner syndrome has been recommended for screening even for patients with no identified cardiovascular defects (13).
Research on the frequency of cardiovascular abnormalities in Korean Turner syndrome patients is insufficient, and there are few studies using radiologic methods other than echocardiography, such as computed tomography or magnetic resonance angiography. Therefore, the authors have studied the frequency of cardiovascular abnormalities among asymptomatic Korean Turner syndrome adolescents using multidetector computed tomography (MDCT).
This study included adolescents aged 13 to 22 yr (average 15.7±2.6 yr), who had Turner syndrome confirmed by chromosome analysis, recruited from June 1995 to August 2004 at the endocrinology clinic of Busan Paik Hospital. A total of 20 subjects, who had no cardiac symptoms, were included.
Blood pressure, electrocardiograph, and MDCT were measured for each subject on visits from August 2007 to February 2009. Hypertension was defined as having both a systolic and diastolic blood pressure over the 95th percentile for age, gender, and height for Korean children and adolescents (14). Hypotension was defined as less than the 5th percentile for age, gender, and height for Korean children and adolescents. Cardiovascular abnormalities were evaluated by MDCT (Aquilion 64, Toshiba Medical System, Japan), and restructuring of the image was performed with a 3D-image restructuring program (Rapidia, Version 2.8, Infinitt Co., Seoul, Korea). Image analysis of cardiac structure, valvular anomaly, aortic arch, and major thoracic vessel anomalies were interpreted by one specialist. In our study, abnormal dilatation of the subclavian artery was defined as a 50% increase over the normal diameter, as like the definition of aortic aneurysm (15). The aortic root dilatation was defined as larger than 2.1 cm/m2 for the aortic diameter at the sinuses (16).
The average age at diagnosis was 9.3±2.9 yr (4-14 yr), and the average observation period was 4.7±2.3 yr. The patients were classified by chromosome karyotype: 8 patients with 45,X (40%), 8 (40%) with mosaicism, and 4 (20%) with X chromosome structural abnormality (Table 1).
The average mean systolic blood pressure was 97±8 mmHg, the mean diastolic blood pressure was 61±5 mmHg, and the mean aortic blood pressure was 73±5 mmHg. No patient had hypertension or hypotension.
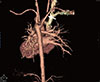
In MDCT, all subject had a left aortic arch. Abnormalities commonly found in Turner syndrome, such as aortic coarctation, bicuspid aortic valve, and aortic aneurysm, were not found. Six (30%) of 20 patients had arterial abnormalities. An aberrant right subclavian artery (Fig. 1) was the most common (3 patients, 15%), and dilatation of the left subclavian artery was found in 2 patients (10%). One patient each (5%) had aortic root dilatation (Fig. 2), aortic diverticulum, left vertebral artery, and other anomalies (Table 2).
Venous malformations also founded in MDCT. Partial anomalous pulmonary venous return (PAPVR) was the most common (3 patients, 15%, Fig. 3), and the pulmonary veins from the left upper lobe were most commonly connected to the left brachiocephalic vein. Other than these anomalies, 2 patients (10%) had a persistent left superior vena cava (Table 2, Fig. 4).
It is well known that congenital cardiovascular abnormalities are frequent in Turner syndrome patients (3-5).
According to other researches, the frequency of congenital cardiovascular abnormalities was reported to be 23% to 45% in Turner syndrome (3, 6), and aortic coarctation and a bicuspid aortic valve have been reported as the most common cardiovascular abnormalities. The cause of cardiovascular abnormalities in Turner syndrome is still not clearly understood, but the main hypothesis is that these cardiac defects may be associated with an increase in a lymphatic obstruction during embryogenesis, which will cause decreased blood flow to the left side of the heart (20, 21). These hypotheses show the high variability of congenital cardiovascular abnormalities in patients with a webbed neck and have earned support (7, 20).
The most common congenital cardiovascular abnormality in Turner syndrome patients is a bicuspid aortic valve with a prevalence of 13%-34% (6), and Korean research shows a rate of 7.8% (22). However, this study did not find any patients with a bicuspid aortic valve. This result likely represents racial variations. Additionally, compared to previous Korean reports (22), the prevalence of a bicuspid aortic valve or aortic coarctation was low in this study. This difference was probably caused by previous reports including Turner syndrome patients diagnosed with cardiovascular abnormalities during infancy, while this study included only Turner syndrome patients without cardiovascular symptoms. Additionally, the small sample size in this study could be a factor.
Bicuspid aortic valve, aortic coarctation, and hypertension are risk factors for aortic dissection; therefore, an evaluation of the cardiovascular system in Turner syndrome is essential (7, 23). Hirst et al. (24) reported that among patients with aortic dissection, 9%-23% had aortic coarctation, 23%-42% had a bicuspid aortic valve, and 63% had hypertension. Allen et al. (25) and Dawson-Falk et al. (3) reported an increase in the aortic root diameter in Turner syndrome patients compared to the control group, and this result also predicts the increased risk of aortic dissection risk in Turner syndrome. This study did not include patients with hypertension or cardiovascular abnormalities, such as bicuspid aortic valve or aortic coarctation, which are commonly found in Turner syndrome patients, but cardiovascular abnormalities, such as aortic root dilatation or aortic diverticulum, were found. This result also needs longitudinal evaluation because the future aortic dissection risk increases in Turner syndrome patients. Additionally, an evaluation for hypertension with increasing age is necessary.
The prevalence of a persistent left superior vena cava in previous research on Turner syndrome patients was 5% to 13% (3, 8). Prandstraller et al. (26) and Lee et al. (22) reported partial anomalous pulmonary venous return in less than 3% of Turner syndrome patients. This study found a persistent left superior vena cava in 13.3% and a partial anomalous pulmonary venous return in 20%, which is a higher frequency of venous abnormalities compared to previous research. Due to the sample size, these results cannot be generalized, but the increased frequency probably contributes to the development of radiology technology that allows a higher detection rate of vessel abnormalities.
Similar to previous studies, this research did not include cases of Turner syndrome patients with a partial anomalous pulmonary venous return and an atrial septal defect (10-12, 27), and most of the venous malformations in this study were partial anomalous pulmonary venous return. These patients did not have clinical symptoms. However, Price et al. (28) reported a secondary congestive heart failure due to a partial anomalous pulmonary venous return in 2 Turner syndrome patients. Therefore, serial evaluation for cardiovascular disorders is necessary, even if no symptoms are present.
We used cardiac MDCT to diagnose the cardiac abnormalities in Turner syndrome. However, MRI is widely available in Turner syndrome recently and recommended for older girls and adults with Turner syndrome, particularly at transition period to adult (13). Cardiac MRI is outstanding to detect degrees of aortic dilatation and coarctation that are not visible on echocardiography (3), but is limited by its high cost and poor tolerability due to claustrophobia in some Turner syndrome patient. Meanwhile, fast scan seeds and low radiation dose and increased anatomic coverage are improving the image quality of cardiac MDCT and reducing patient risks in children (29). Cardiac MDCT is also considered that it can effectively bridge the gaps among echochardiography and cardiac MRI in children with congenital heart disease (30). In addition, cardiac MDCT has cost benefit compare with cardiac MRI in Korea. We could not found aortic coarctation in our patients, but found arotic root dilatation and aortic diverticulum as well as venous anomaly using cardiac MDCT.
This study evaluated cardiovascular malformations in Turner syndrome patients without cardiac symptoms at adolescence. Generalizability is not possible due to the limited number of subjects, but a high frequency of cardiovascular abnormalities was found in this study. These vessel anomalies can increase the risk of future aortic dissection or secondary heart failure. Therefore, although patients may have no cardiac symptoms or no abnormal findings on echocardiography at diagnosis, reevaluation of cardiovascular system using radiological tests are necessary during adolescence and longitudinal observation is needed. In addition, we found that not only MRI but also MDCT can be a good modality to detect hidden cardiovascular anomalies, which are difficult to detect by echocardiography. Especially in patients with cardiovascular abnormalities and aortic dissection risk factors, a regular evaluation and active treatment of cardiovascular disease risk factors are crucial.
Figures and Tables
Fig. 1
An aberrant right subclavian artery arising from the descending side of the aortic arch (arrow).
References
1. Gravholt CH. Epidemiological, endocrine and metabolic features in Turner syndrome. Eur J Endocrinol. 2004; 151:657–687.
2. Bondy CA, Bakalov VK. Investigation of cardiac status and bone mineral density in Turner syndrome. Growth Horm IGF Res. 2006; 16:S103–S108.
3. Dawson-Falk KL, Wright AM, Bakker B, Pitlick PT, Wilson DM, Rosenfeld RG. Cardiovascular evaluation in Turner syndrome: utility of MR imaging. Australas Radiol. 1992; 36:204–209.
4. Lemli L, Smith DW. The XO syndrome: a study of the differentiated phenotype in 25 patients. J Pediatr. 1963; 63:577–588.
5. Rainier-Pope CR, Cunningham RD, Nadas AS, Crigler JF Jr. Cardiovascular malformation in Turner's syndrome. Pediatrics. 1964; 33:919–925.
6. Mazzanti L, Cacciari E. Congenital heart disease in patients with Turner's syndrome: Italian Study Group for Turner Syndrome (ISGTS). J Pediatr. 1998; 133:688–692.
7. Lin AE, Lippe B, Rosenfeld RG. Further delineation of aortic dilation, dissection, and rupture in patients with Turner syndrome. Pediatrics. 1998; 102:e12.
8. Ho VB, Bakalov VK, Cooley M, Van PL, Hood MN, Burklow TR, Bondy CA. Major vascular anomalies in Turner syndrome: prevalence and magnetic resonance angiographic features. Circulation. 2004; 110:1694–1700.
9. Mazzanti L, Prandstraller D, Tassinari D, Rubino I, Santucci S, Picchio FM, Forabosco A, Cacciari E. Heart disease in Turner's syndrome. Helv Paediatr Acta. 1988; 43:25–31.
10. Moore JW, Kirby WC, Rogers WM, Poth MA. Partial anomalous pulmonary venous drainage associated with 45,X Turner's syndrome. Pediatrics. 1990; 86:273–276.
11. Van Wassenaer AG, Lubbers LJ, Losekoot G. Partial abnormal pulmonary venous return in Turner syndrome. Eur J Pediatr. 1988; 148:101–103.
12. Subramaniam PN. Turner's syndrome and cardiovascular anomalies: a case report and review of the literature. Am J Med Sci. 1989; 297:260–262.
13. Bondy CA. Turner Syndrome Study Group. Care of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab. 2007; 92:10–25.
14. Lee CG, Moon JS, Choi JM, Nam CM, Lee SY, Oh K, Kim YT. Normative blood pressure references for Korean children and adolescents. Korean J Pediatr. 2008; 51:33–41.
15. Johnston KW, Rutherford RB, Tilson MD, Shah DM, Hollier L, Stanley JC. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg. 1991; 13:452–458.
16. Roman MJ, Devereux RB, Kramer-Fox R, O'Loughlin J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol. 1989; 64:507–512.
17. Apple J, McQuade KL, Hamman BL, Hebeler RF, Shutze WP, Gable DR. Initial experience in the treatment of thoracic aortic aneurysmal disease with a thoracic aortic endograft at Baylor University Medical Center. Proc (Bayl Univ Med Cent). 2008; 21:115–119.
18. Ho ML, Bhalla S, Bierhals A, Gutierrez F. MDCT of partial anomalous pulmonary venous return (PAPVR) in adults. J Thorac Imaging. 2009; 24:89–95.
19. Imran N, Grubb B, Kanjwal Y. Persistent left superior vena cava: a blessing in disguise. Europace. 2008; 10:588–590.
20. Clark EB. Neck web and congenital heart defects: a pathogenic association in 45 X-O Turner syndrome? Teratology. 1984; 29:355–361.
21. Lacro RV, Jones KL, Benirschke K. Coarctation of the aorta in Turner syndrome: a pathologic study of fetuses with nuchal cystic hygromas, hydrops fetalis, and female genitalia. Pediatrics. 1988; 81:445–451.
22. Lee MK, Jhang WK, Ko JM, Kim YH, Ko JK, Yoo HW, Park IS. Congenital cardiovascular malformations in patients with Turner syndrome. J Korean Pediatr Cardiol Soc. 2006; 10:292–298.
23. Sybert VP. Cardiovascular malformations and complications in Turner syndrome. Pediatrics. 1998; 101:E11.
24. Hirst AE Jr, Johns VJ Jr, Kime SW Jr. Dissecting aneurysm of the aorta: a review of 505 cases. Medicine (Baltimore). 1958; 37:217–279.
25. Allen DB, Hendrick SA, Levy JM. Aortic dilation in Turner syndrome. J Pediatr. 1986; 109:302–305.
26. Prandstraller D, Mazzanti L, Picchio FM, Magnani C, Bergamaschi R, Perri A, Tsingos E, Cacciari E. Turner's syndrome: cardiologic profile according to the different chromosomal patterns and long-term clinical follow-Up of 136 nonpreselected patients. Pediatr Cardiol. 1999; 20:108–112.
27. Shiroma K, Ebine K, Tamura S, Yokomuro M, Suzuki H, Takanashi Y. A case of Turner's syndrome associated with partial anomalous pulmonary venous return complicated by dissecting aortic aneurysm and aortic regurgitation. J Cardiovasc Surg (Torino). 1997; 38:257–259.
28. Price WH, Willey RF. Partial anomalous pulmonary venous drainage in two patients with Turner's syndrome. J Med Genet. 1980; 17:133–134.
29. Goo HW. State-of-the-art CT imaging techniques for congenital heart disease. Korean J Radiol. 2010; 11:4–18.
30. Goo HW. Cardiac MDCT in children: CT technology overview and interpretation. Radiol Clin North Am. 2011; 49:997–1010.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download