Abstract
Interleukin (IL)-33 is an important mediator of innate immunity. Behcet's disease (BD) is an autoinflammatory disorder characterized by hyperactivity of the innate immune response. We measured serum levels of IL-33 and its receptor soluble ST2 (sST2) in patients with BD to investigate their association with disease activity. Serum levels of both IL-33 and sST2 were higher in patients with BD compared with those in normal controls (IL-33: 594.48±175.04 pg/mL in BD and 224.23±56.64 pg/mL in normal controls [P=0.048], sST2: 99.01±15.92 pg/mL in BD and 23.56±3.25 pg/mL in normal controls [P<0.001]). IL-33 and sST2 expression in skin tissue, as shown by immunohistochemistry, was higher in patients with BD compared with that in the normal controls. Serum sST2 level correlated significantly with the BD currently active form (BDCAF), Iranian BD dynamic activity measure (IBDDAM), erythrocyte sedimentation rate and C-reactive protein. Multiple linear regression showed that serum sST2 was an independent factor associated with IBBDAM (regression coefficient, 0.374; P=0.004), and BDCAF (regression coefficient, 0.236; P=0.047). These results demonstrate that IL-33 and sST2 are highly expressed in patients with BD and that serum sST2 is an independent factor associated with IBDDAM and BDCAF, suggesting a potential role for sST2 as a surrogate marker of disease activity in patients with BD.
Interleukin 33 (IL-33), a recently identified member of the IL-1 family, is a ligand for the orphan receptor ST2 (also known as IL-1RL1). When IL-33 binds to ST2, it enhances inflammatory cytokines by activating nuclear factor-κB (NF-κB) and mitogen activated protein (MAP) kinases (1). IL-33 is mainly expressed by endothelial cells, fibroblasts, epithelial cells, keratinocytes, and is particularly high in endothelial venules (2). ST2 is the receptor for IL-33 and a member of the IL-1 receptor family. ST2 exists as either a membrane bound form (ST2L) or as a soluble form (sST2). ST2L functions as a transmembrane signaling receptor for IL-33 by mediating the effect of IL-33 on the inflammatory process. sST2 is a soluble receptor that can suppress IL-33 activity (3).
Both enhanced innate immunity and neutrophil hyperactivity with endothelial damage, which are part of BD pathogenesis, are also related to elevated IL-33 levels. Therefore, we hypothesized that BD may be associated with changed IL-33/sST2 levels, which may be involved in BD pathogenesis. Therefore, we analyzed serum IL-33 and sST2 levels in patients with BD and its correlation with other clinical parameters.
Fifty-three unrelated Korean patients with BD (15 men and 38 women, mean age, 41±12 yr) were recruited from the rheumatology clinics, Seoul St. Mary's Hospital which are affiliated with the medical school of The Catholic University of Korea. This study was approved by the Research Ethics Board of the Medical School, The Catholic University of Korea. All patients with BD recruited for this study met the 1990 international criteria for classification of BD. In addition, this study included 31 age- and sex-matched healthy individuals, unrelated to the patients (7 men and 24 women, mean age 43±16 yr), without inflammatory or autoimmune diseases. Blood samples were drawn from the 53 patients with BD and 31 normal controls (NC). All individuals gave informed consent. All patients with BD underwent routine laboratory assessments at their clinic visit. The specific clinical manifestations referred to manifestations at the time of the study, and the Behcet's disease current activity form (BDCAF) (4) and Iranian Behcet's disease dynamic activity measure (IBDDAM) score (5) were determined for each patient at the time of the blood draw.
Serum IL-33 and sST2 levels were measured using a sandwich double monoclonal antibody enzyme-linked immunosorbent assay method according to the manufacturer's directions (R&D Systems, Minneapolis, MN, USA).
Skin biopsy specimens were obtained with informed consent from five patients in the active BD group who underwent a diagnostic evaluation of skin lesions indicative of BD. Among the biopsies, four were diagnosed with erythema nodosum (EN)-like skin lesions and the other one was EN. Three NC skin tissues were obtained from pathologists. The main reason for the skin biopsy was the obscure skin lesions observed by dermatologists. All NC skin specimens were diagnosed as normal by pathologists. Standard paraffin specimens were prepared, sectioned at 5-µm thickness, and stained with hematoxylin and eosin. The slides were examined for the presence of panniculitis by two experienced pathologists. Immunohistochemistry was performed on serial sections using an indirect immunoperoxidase method, and the paraffin-embedded slides were deparaffinized by immersion in xylene, followed by dehydration in ethanol. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide. The sections were incubated in a blocking solution for 30 min at room temperature and then overnight at 4℃ with primary antibodies against IL-33 and sST2 (R&D Systems). The slides were washed for 5 min, followed by a 20-min incubation with biotinylated secondary antibodies (Vector Labs., Burlingame, CA, USA). After a 15 min wash, the slides were incubated for 1 hr with avidin-conjugated horseradish peroxidase using the Vecterstain ABC Elite (Vector Labs.). Samples were photographed with a photomicroscope (Leica, Wetzlar, Germany).
Means±standard errors were used to describe IL-33 and sST2 concentrations and other laboratory values. Serum concentrations of IL-33 and sST2 were compared between patients with BD and the NC using the unpaired t-test. The associations between baseline clinical or laboratory characteristics and serum IL-33 and sST2 levels were analyzed with the Mann-Whitney U-test for categorical variables and Pearson's correlation coefficient for continuous variables. Multiple linear regression was used to estimate the independent association of IL-33 and sST2 levels with the variables to exclude the effects of other related variables on the results. All analyses were performed using SAS software version 9.0 (SAS Institute, Cary, NC, USA). A P<0.05 was considered significant.
Fifty-three patients with BD and 31 age- and sex-matched NC were recruited. The demographic and clinical parameters are shown in Table 1. Of the 53 patients with BD, the most frequent symptom was oral ulcer (100%). Other symptoms included genital ulcers (66%), cutaneous lesions (57%), arthritis (42%), uveitis (36%), gastrointestinal (GI) tract involvement (13%), central nervous system (CNS) involvement (8%), and thrombosis (6%). The median scores for the BDCAF and IBDDAM were 2.53 and 1.87, respectively.
Serum levels of IL-33 and sST2 were measured in the 53 patients with BD and the 32 age- and sex-matched NC. As shown in Fig. 1, the mean levels of IL-33 were 594.5±175.04 pg/mL (range, 0.6-5,632.9 pg/mL) and 244.2±56.64 pg/mL (range, 0.1-1,389.5 pg/mL) in sera, respectively, from patients with BD and the NC. The mean levels of sST2 were 99.0±15.92 pg/mL (range, 3.0-552.3 pg/mL) and 23.6±3.25 pg/mL (range, 1.8-87.2 pg/mL) in sera, respectively, from patients with BD and the NC. The differences in the serum levels of IL-33 and sST2 between the patients with BD and the NC were statistically significant (P=0.048, P<0.001, respectively).
IL-33 and sST2 immunohistochemistry was performed on the skin lesions from patients with BD and the NC to evaluate whether IL-33 and sST2 increased in affected skin lesions. The skin lesions of the patients with BD were diagnosed as EN or EN-like skin lesions and were confirmed by dermatologists and pathologists. As shown in Fig. 2, the affected dermal skin tissues from patients with BD had septal panniculitis with thickened connective tissue septa of the subcutis compared with that of normal skin tissues. They also demonstrated numerous aggregates of lymphocytes and neutrophils interstitially arranged between collagen bundles of the septa and fat lobules, which are typical of BD related EN-like skin lesions (Fig. 2A, B). IL-33 was expressed in endothelial cells in dermal vessels and fibroblasts in the thickened connective tissue septa of the subcutis compared with that in the NC skin samples (Fig. 2C, D). Similarly, sST2 was overexpressed in BD skin lesions, compared with that in the NC skin samples (Fig. 2E, F). Furthermore, similar to the findings in dermal layers, IL-33 and sST2 overexpression was observed in epidermal skin layers from patients with BD compared to that of NC skin samples (Fig. 3). IL-33 and sST2 immunoreactivity was observed in epidermal layers, not only in nuclei but also in the cytoplasm in the epidermal keratinocytes and endothelial cells of dermal vessels in EN or EN-like skin lesions of patents with BD. Similar IL-33 and sST2 immunoreactivity was observed in the nuclei of the NC skin specimens, but reduced IL-33 and sST2 immunoreactivity was observed in the cytoplasm of the epidermis layer and dermal vessels (Fig. 3A-D). These results indicate that IL-33 and sST2 were expressed more in the cytoplasm in epidermal keratinocytes and endothelial cells of dermal vessels in the BD skin lesions than those in the NC skin specimens.
Next, we analyzed the association between serum IL-33 levels and age, gender, onset age, and disease duration. As shown in Tables 2 and 3, serum IL-33 did not significantly correlate with age in patients with BD (r=-0.190 , P=0.173) or the NC (r=-0.288, P=0.116) and did not differ significantly between women and men in patients with BD (P=0.147) or the NC (P=0.179). IL-33 did not significantly correlate with onset age or disease duration in patients with BD (r=-0.272; P=0.071, r=0.138; P=0.365, respectively). We also analyzed the association between serum sST2 levels and age, gender, onset age, and disease duration. As shown in Tables 2 and 3, serum sST2 levels did not significantly correlate with age in patients with BD (r=-0.190; P=0.173) or the NC (r=0.019; P=0.920) and did not differ significantly between women and men in patients with BD (P=0.070) or the NC (P=0.562). Additionally, sST2 did not significantly correlate with onset age or disease duration in patients with BD (r=-0.227; P=0.133, r=0.164; P=0.282, respectively).
As shown in Table 2, serum IL-33 levels were related to skin lesions (P=0.011) but not other organ involvement including genital ulcers (P=0.095), uveitis (P=0.375), arthritis (P=0.505), thrombosis (P=0.675), CNS involvement (P=0.191), or gastrointestinal tract (GI) tract involvement (P=0.207). Patients with skin lesions had higher serum IL-33 levels (774.0±224.8 pg/mL) compared with those without skin lesions (360.3±243.8 pg/mL) (P=0.011). Serum sST2 levels were related to thrombosis (P=0.035) and GI tract involvement (P=0.037). But, serum sST2 level was not related to genital ulcers (P=0.735), uveitis (P=0.528), skin lesions (P=0.110), arthritis (P=0.364), or CNS involvement (P=0.910). Patients with thrombosis had higher serum sST2 levels (223.9±90.3 pg/mL) compared with those without thrombosis (91.5±15.7 pg/mL) (P=0.035). Patients with GI tract involvement had higher serum sST2 levels (172.7±66.9 pg/mL) compared with those without GI tract involvement (87.8±15.0 pg/mL) (P=0.037).
As shown in Table 2, serum IL-33 level was not related to medications including prednisolone (P=0.825), colchicine (P=0.497), azathioprine (P=0.901), cyclosporin (P=0.323), or sulfasalazine (P=0.894). Serum ST2 level was related to colchicine (P=0.001), but was not related to other medications including prednisolone (P=0.343), azathioprine (P=0.901), cyclosporin (P=0.871), or sulfasalazine (P=0.333). Patients taking colchicine had lower serum ST2 levels (74.5±13.3 pg/mL) compared with those not taking the medication (146.6±37.3 pg/mL) (P=0.001).
Serum IL-33 level was not correlated with sST2 level in patients with BD (r=-0.096; P=0.49) or the NC (r=0.011; P=0.95). But, as shown in Table 3 and Fig. 4, a significant positive correlation was found between serum sST2 and acute phase reactants (erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP]) (ESR, r=0.389; P=0.004; CRP, r=0.445; P=0.002). Additionally, serum sST2 levels in patients with BD were significantly correlated with BD activity scores (BDCAF and IBDDAM) (BDCAF, r=0.453; P=0.001; IBDDAM, r=0.514; P<0.001). In contrast, serum IL-33 levels did not correlate with the level of acute phase reactants (ESR and CRP) (ESR, r=-0.3; P=0.79 ; CRP, r=-0.08; P=0.55) and BD activity score (BDCAF and IBDDAM) (BDCAF, r=-0.03; P=0.82; IBDDAM, r=-0.5; P=0.67).
We showed that sST2 level was correlated with thrombosis, GI tract involvement, current colchicine use, disease activity score (BDCAF and IBDDAM), and acute phase reactants (ESR and CRP). A multiple linear regression analysis was conducted using disease activity score (BDCAF and IBDDAM) as a dependent variable and serum ST2 levels, ESR, CRP, and daily colchicine dose as independent variables (Table 4). As shown in Table 4, the multiple linear regression revealed that both serum CRP and sST2 were independent predictors for IBBDAM (CRP: regression coefficient=0.616; P=0.001; sST2: regression coefficient=0.374; P=0.004), and BDCAF (CRP: regression coefficient=0.733; P=0.000; sST2: regression coefficient=0.236; P=0.047).
Increasing evidence has shown that IL-33 and its receptor ST2 contribute to the pathogenesis of various autoimmune diseases. However, no information is available on the role of IL-33 and sST2 in BD in comparison to other autoimmune diseases. In this study, we found that serum IL-33 and sST2 levels were elevated in patients with BD compared to those in the NC. In particular, serum sST2 was positively correlated with acute phase reactants (ESR and CRP) and BD activity scores (BDCAF and IBDDAM). Moreover, we found that IL-33 and sST2 were highly expressed in the epidermis and dermis of the skin of patients with BD compared to that of the NC. This result was consistent with that in previous studies, indicating that serum IL-33 and sST2 protein levels are elevated and correlated with disease activity markers in other rheumatologic diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), ankylosing spondylitis (AS), and vasculitis (6-9).
Yang et al. (10) reported that IL-33 increases significantly in patients with SLE compared to that in controls, but that most clinical and laboratory characteristics do not correlate with serum IL-33 levels. They suggested that IL-33 might play a role in the acute phase of SLE, but it was not associated with the disease course. In another study in SLE, serum sST2 is significantly higher in patients with active SLE compared with that in inactive patients and NCs and that serum sST2 level was correlated significantly with the SLE Disease Activity Index (SLEDAI) and anti-dsDNA levels (8). They also found that serum sST2 level is an independent predictive factor for the modified SLEDAI and is sensitive to longitudinal changes in disease activity. Other investigations have also indicated a similar relationship between the IL-33/ST2 system and RA, wherein the levels of serum and synovial fluid (SF) IL-33 and sST2 are significantly higher in patients with RA than those in controls (2, 3, 11, 12). Hong et al. (13) reported that serum levels of IL-33 and sST2 are correlated with IL-1β and IL-6. In particular, serum IL-33 level is correlated with CRP, DAS28-CRP, rheumatoid factor (RF), and anti-citrullinated protein antibody (14, 15). In contrast, silencing IL-33 significantly reduces tumor necrosis factor-α (TNF-α)-induced synthesis of IL-33, IL-6, IL-8, and monocyte chemotactic protein-1 in RA-SF at the mRNA and protein levels (16). Furthermore, antirheumatic drugs treatments such as disease-modifying antirheumatic drugs and etanercept reduce serum levels of IL-33, sST2, and CRP in patients with RA (13-15). Serum IL-33 levels are also elevated in patients with AS compared to that in controls and that serum IL-33 is significantly correlated with TNF-α, IL-17, and BASDAI score in patients with AS (7). In patients with vasculitis, serum IL-33 level is elevated in Henoch Schönlein purputa (HSP) and is positively correlated with clinical activity score, IgA level (17). Moreover, tissue IL-33 level is elevated in giant cell arteritis (18). Serum sST2 level is also elevated in Wegener's granulomatosis (WG) (9). As shown in these studies, the IL-33/ST2 system is activated in various autoimmune diseases because serum IL-33 and/or sST2 levels were elevated and the IL-33/ST2 system was correlated with disease activity markers.
In this study, we observed higher serum levels and tissue expression of IL-33 in patients with BD compared with those in the NC. However, serum IL-33 levels were not correlated with inflammatory markers, such as acute phase reactants or BD activity scores. Hence, these data suggest that IL-33 may partly contribute to the pathogenesis of BD but is not a disease activity marker. In some studies of patients with SLE, serum IL-33 levels were not correlated to inflammation (ESR and CRP) or disease activity (SLEDAI), which was consistent with our results (8, 10). But, our data were not consistent with previous RA, AS, and HSP studies, showing that serum IL-33 levels are correlated with CRP and DAS28-CRP in patients with RA, in patients with AS, and also correlated with IgA level in HSP (7, 14, 17). No study has explained the reason for the inconsistent correlation between IL-33 levels and disease activity in various autoimmune diseases. Therefore, further studies are needed to define the role of elevated IL-33 levels in the context of disease activity of autoimmune diseases.
In our study, serum and tissue expression of sST2 in patients with BD was higher than those in normal controls. Moreover, unlike IL-33, serum sST2 level was correlated with acute phase reactants and BD activity scores. In particular, serum sST2 levels remained significantly associated with BD activity score (IBDDAM and BDCAF) after multiple linear regression, demonstrating higher sST2 levels among patients with BD and active disease compared with those with less disease activity. These BD data are consistent with previous SLE studies, showing that serum sST2 levels are correlated significantly with SLEDAI and anti-dsDNA level (8). Overall, our data suggest that serum sST2, unlike IL-33, may not only partly contribute to the pathogenesis of BD but could also be a disease activity marker.
One of the limitations of our study was that we did not determine the cause of elevated serum IL-33 levels in patients with BD. But, abundant expression of IL-33 mRNA is observed in endothelial cells from inflamed human tonsils, the intestines of patients with Crohn's disease, and rheumatoid synovium (19). In a recently reported RA study, TNF-α significantly induced IL-33 mRNA expression and protein synthesis in RA-synovial fibroblasts via p38 signaling, and IL-33 overexpression significantly increased TNF-α-induced IL-6, IL-8, and matrix metalloproteinase-3 in RA-synovial fibroblasts (16). Additionally, increased TNF-α levels are found in patients with BD, which supports the empiric use of anti-TNF-α therapies (20). Hence, elevated TNF-α level may, in part, be the cause of the elevated IL-33 level in patients with BD. Furthermore, endothelial damage in patients with BD might allow IL-33 to be released as an 'alarmin'. Therefore, elevated IL-33 levels may be partly caused by vasculitis in patients with BD (2). In support of this, IL-33 was highly expressed in dermal fibroblasts, capillary endothelial cells, epidermal keratinocytes, and epithelial cells of skin from patients with BD. These cells abundantly express IL-33 and constitute a major source of IL-33 mRNA in chronically inflamed tissues (19). IL-33 localizes only to the nucleus of endothelial cells, epithelial cells, fibroblasts, keratinocytes, and smooth muscle cells in the absence of proinflammatory stimuli. But, under conditions such as infection or inflammation, IL-33 expression is enhanced and cytoplasm-nuclear redistribution of IL-33 occurs following pro-inflammatory stimulation (TNF-α and IL-1) and is released following cell lysis as a function of "alarmins" (2). Therefore, the cause of the increased serum IL-33 level in patients with BD was suspected to be due to damage of endothelial and epithelial cells by the vasculitis process.
sST2 is a soluble receptor that is different from the membrane-bound form of the receptor (ST2L). The upregulation of soluble cytokine receptors is not fully understood, but sST2 expression also increases following pro-inflammatory stimulation (TNF-α, IL-1, or lipopolysaccharides) (21). sST2 was highly expressed with IL-33 in dermal fibroblasts, capillary endothelial cells, and epidermal keratinocytes in the skin of patients with BD. The pathogenesis of the elevated serum and tissue sST2 in patients with BD remains speculative, but we suggest that sST2 may increase in patients with BD for the following reasons: 1) Increased inflammatory cytokine (TNF-α and IL-1) -induced expression of sST2 in tissue. 2) The sST2 in tissue was released following cell lysis due to tissue damage by vasculitis in patients with BD. The function of sST2 is not completely clear, but, recent reports have suggested that the sST2 protein could be involved in the inflammatory response as well as in Th2 immune responses (21). ST2L mediates the effect of the IL-33-induced inflammatory process, whereas sST2 functions as a negative regulator and antagonistic decoy receptor for IL-33 (3). IL-33 has been identified as a ligand for ST2L, a marker of Th2 T lymphocytes. ST2L is involved in the regulation of the Th2-associated immune response at the effector stage and in Th2 driven immunopathology. The interaction between IL-33 and ST2L leads to the induction of the Th2 cytokines IL4, IL-5, and IL-13 through a signaling mechanism that involves activation of NF-κB and MAP kinases (1). Otherwise, sST2 could act as an anti-inflammatory mediator, through a mechanism that involves inhibiting Toll-like receptor signaling by sequestering MyD88 and Mal adapter proteins or inhibiting I-κB degradation resulting in downregulation of NF-κB (21).
In vitro and in vivo experiments have shown that the sST2 protein or an ST2-fusion protein attenuates production of the pro-inflammatory cytokines IL-1β, TNF-α, IL-6, and IL-12 (22, 23). High levels of pro-inflammatory cytokines such as TNF-α and IL-6 have been found in patients with BD (20) and these cytokines induce sST2 (21). Hence, we suggest that elevated sST2 levels found in patients with BD could be an indication of immune hyperactivation, and/or a mechanism to downregulate or attenuate immune hyperactivation that occurs in patients with BD.
The antagonist effect of sST2 on inflammatory signaling has been described in other rheumatic diseases such as SLE and RA. Serum levels of sST2 increase in patients with SLE and are correlated with parameters of lupus disease activity. An inverted correlation has been reported between sST2 and C3 levels in patients with lupus, indicating a potential regulatory role for sST2 in the disease (8). In a mouse model of collagen-induced arthritis, the sST2-Fc fusion protein significantly reduces cellular infiltration, synovial hyperplasia, and joint erosion in joints and also downregulates serum levels of IL-6, IL-12, and TNF-α (23). In a mouse model of ischemia/reperfusion, pre-treatment with the sST2-Fc fusion protein decreases the inflammatory response (24).
Overall, the findings of our study showed elevated serum levels of IL-33 and sST2 in patients with BD and a correlation between sST2 and disease activity. This finding suggests that the IL-33/ST2 system may have a role in the pathogenesis of BD. The role of IL-33 in BD is of interest because of its role as an alarmin and the role of sST2 as a marker of inflammation in patients with BD. In view of the significant correlation between serum sST2 level, disease activity parameters, and the responsiveness of sST2 to changes in disease activity when monitored longitudinally, sST2 could serve as a potential surrogate marker for BD disease activity. However, as we could only demonstrate a clinical association rather than a cause for the increase in sST2, further studies are required to delineate the role of sST2 as a biomarker in BD, or as a regulatory mechanism involved in disease pathogenesis. Further studies on the functional role of the IL-33/ST2 axis in BD pathogenesis may result in more therapeutic options for these patients.
Figures and Tables
Fig. 1
Serum levels of interleukin (IL)-33 and receptor soluble ST2 (sST2) between patients with BD and NC. Serum levels of IL-33 and sST2 were significantly higher in patients with BD than those in the NC. BD, Behcet's disease; NC, Normal control.
Fig. 2
IL-33 and sST2 expression in the skin (dermis). (A, B) H&E stain. Septal panniculitis existed in the skin of patients with Behcet's disease (BD). (C-F) IHC stain. IL-33 and sST2 were highly expressed in the dermis of skin from patients with BD. H&E, hematoxylin & eosin; IHC, immunohistochemical.
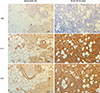
Fig. 3
IL-33 and sST2 expression in the skin (epidermis). (A-D) IHC stain. IL-33 and sST2 were highly expressed in the epidermis of skin from patients with BD. IHC, immunohistochemical.
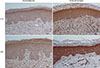
Fig. 4
Correlation between receptor soluble ST2 (sST2) and disease activity parameters in patients with Behcet's disease (BD). sST2 was correlated significantly with acute phase reactant (erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP]) (ESR: r=0.389, P=0.004; CRP: r=0.445, P=0.002). sST2 was correlated significantly with Behcet's disease activity score (BD currently active form [BDCAF] and Iranian BD dynamic activity measure [IBDDAM]) (BDCAF: r=0.453, P=0.001; IBDDAM: r=0.514, P<0.001).

Table 1
Cumulative demographic features and clinical manifestations at the time of study in the patients with Behcet's disease and the normal controls

Table 2
Association between serum interleukin-33 (IL-33) and receptor soluble ST2 (sST2) levels and clinical data in patients with Behcet's disease (BD)

Table 3
Correlation analysis between serum interleukin-33 (IL-33) and receptor soluble ST2 (sST2) and clinical data in patients with Behcet's disease (BD)
Notes
References
1. Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005; 23:479–490.
2. Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'? PLoS One. 2008; 3:e3331.
3. Bergers G, Reikerstorfer A, Braselmann S, Graninger P, Busslinger M. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO J. 1994; 13:1176–1188.
4. Bhakta BB, Brennan P, James TE, Chamberlain MA, Noble BA, Silman AJ. Behçet's disease: evaluation of a new instrument to measure clinical activity. Rheumatology (Oxford). 1999; 38:728–733.
5. Shahram F, Khabbazi A, Nadji A, Ziaie N, Banihashemi AT, Davatchi F. Comparison of existing disease activity indices in the follow-up of patients with Behçet's disease. Mod Rheumatol. 2009; 19:536–541.
6. Verri WA Jr, Souto FO, Vieira SM, Almeida SC, Fukada SY, Xu D, Alves-Filho JC, Cunha TM, Guerrero AT, Mattos-Guimaraes RB, et al. IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann Rheum Dis. 2010; 69:1697–1703.
7. Han GW, Zeng LW, Liang CX, Cheng BL, Yu BS, Li HM, Zeng FF, Liu SY. Serum levels of IL-33 is increased in patients with ankylosing spondylitis. Clin Rheumatol. 2011; 30:1583–1588.
8. Mok MY, Huang FP, Ip WK, Lo Y, Wong FY, Chan EY, Lam KF, Xu D. Serum levels of IL-33 and soluble ST2 and their association with disease activity in systemic lupus erythematosus. Rheumatology (Oxford). 2010; 49:520–527.
9. Kuroiwa K, Arai T, Okazaki H, Minota S, Tominaga S. Identification of human ST2 protein in the sera of patients with autoimmune diseases. Biochem Biophys Res Commun. 2001; 284:1104–1108.
10. Yang Z, Liang Y, Xi W, Li C, Zhong R. Association of increased serum IL-33 levels with clinical and laboratory characteristics of systemic lupus erythematosus in Chinese population. Clin Exp Med. 2011; 11:75–80.
11. Oboki K, Ohno T, Kajiwara N, Saito H, Nakae S. IL-33 and IL-33 receptors in host defense and diseases. Allergol Int. 2010; 59:143–160.
12. Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, Nambu A, Abe T, Kiyonari H, Matsumoto K, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A. 2010; 107:18581–18586.
13. Hong YS, Moon SJ, Joo YB, Jeon CH, Cho ML, Ju JH, Oh HJ, Heo YJ, Park SH, Kim HY, et al. Measurement of interleukin-33 (IL-33) and IL-33 receptors (sST2 and ST2L) in patients with rheumatoid arthritis. J Korean Med Sci. 2011; 26:1132–1139.
14. Kageyama Y, Torikai E, Tsujimura K, Kobayashi M. Involvement of IL-33 in the pathogenesis of rheumatoid arthritis: the effect of etanercept on the serum levels of IL-33. Mod Rheumatol. 2012; 22:89–93.
15. Mu R, Huang HQ, Li YH, Li C, Ye H, Li ZG. Elevated serum interleukin 33 is associated with autoantibody production in patients with rheumatoid arthritis. J Rheumatol. 2010; 37:2006–2013.
16. Kunisch E, Chakilam S, Gandesiri M, Kinne RW. IL-33 regulates TNF-α dependent effects in synovial fibroblasts. Int J Mol Med. 2012; 29:530–540.
17. Chen T, Jia RZ, Guo ZP, Cao N, Li MM, Jiao XY. Elevated serum interleukin-33 levels in patients with Henoch-Schönlein purpura. Arch Dermatol Res. 2013; 305:173–177.
18. Ciccia F, Alessandro R, Rizzo A, Raimondo S, Giardina A, Raiata F, Boiardi L, Cavazza A, Guggino G, De Leo G, et al. IL-33 is overexpressed in the inflamed arteries of patients with giant cell arteritis. Ann Rheum Dis. 2013; 72:258–264.
19. Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A. 2007; 104:282–287.
20. Sayinalp N, Ozcebe OI, Ozdemir O, Haznedaroğlu IC, Dündar S, Kirazli S. Cytokines in Behçet's disease. J Rheumatol. 1996; 23:321–322.
21. Mildner M, Storka A, Lichtenauer M, Mlitz V, Ghannadan M, Hoetzenecker K, Nickl S, Dome B, Tschachler E, Ankersmit HJ. Primary sources and immunological prerequisites for sST2 secretion in humans. Cardiovasc Res. 2010; 87:769–777.
22. Oshikawa K, Yanagisawa K, Tominaga Si, Sugiyama Y. ST2 protein induced by inflammatory stimuli can modulate acute lung inflammation. Biochem Biophys Res Commun. 2002; 299:18–24.
23. Leung BP, Xu D, Culshaw S, McInnes IB, Liew FY. A novel therapy of murine collagen-induced arthritis with soluble T1/ST2. J Immunol. 2004; 173:145–150.
24. Yin H, Huang BJ, Yang H, Huang YF, Xiong P, Zheng F, Chen XP, Chen YF, Gong FL. Pretreatment with soluble ST2 reduces warm hepatic ischemia/reperfusion injury. Biochem Biophys Res Commun. 2006; 351:940–946.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download