Abstract
An accumulation of pigment deposits on mucosa, called melanosis or pseudomelanosis, of the small bowel is observed infrequently during endoscopic examination. We describe 6 cases of small bowel pseudomelanosis; the possible etiology of which was chronic iron intake. We observed numerous brown spots in duodenum, jejunum, and terminal ileum during upper and lower endoscopy. Interestingly, all patients have been taking oral iron for several years. Histology showed pigment depositions within macrophages of the lamina propria and a positive Prussian blue stain indicating hemosiderin deposition. Herein, we demonstrate that long term iron therapy may result in pseudomelanosis of small bowel, such as duodenum, jejunum, and ileum.
Intestinal pseudomelanosis is a condition characterized by dark pigmentation of the intestinal mucosa, such as colon, small bowel, and stomach. Among intestinal pseudomelanosis, melanosis coli is the most common and a well known condition, which occurs with the laxative use, especially anthraquinone containing laxatives. The dark brown pigmentation of melanosis coli is actually lipofuscin in macrophages and not melanin (1). Interestingly, extracolonic pseudomelanosis has also been rarely reported in the literature (2, 3) and several etiologies have been suggested for the development of extracolonic pseudomelanosis. However, exact mechanism of its occurrence is unknown. Most commonly reported location of extracolonic pseudomelanosis is the duodenum, although it can also be found on the mucosa of the stomach, jejum or ileum (2-4). In addition, few reports on pseudomelanosis ilei have attributed its cause to chronic oral iron therapy, but the association is still unclear (2, 5). Few reports showed that there are considerable association with end stage renal disease and hypertension (3). Herein, we describe 6 cases of the small bowel pseudomelanosis with possible etiology being chronic iron intake.
The cases included one man and 5 women, with a mean age of 62 yr (median 69.5, range 34-73). Summary on endoscopic findings, associated medical conditions, and concurrent medications of the subjects are shown in Table 1. Five (83%) patients had chronic kidney disease, 2 of whom were undergoing peritoneal dialysis, 5 (83%) patients had hypertension, and 3 (50%) patients had diabetes mellitus. Two patients had gastrointestinal symptoms, and others were referred for surveillance endoscopy. Various classes of antihypertensive medication including beta blockers, thiazides, angiotensin receptor blockers, and calcium channel blockers were being taken by the hypertensive patients. Other common medications included statin (HMG-CoA reductase inhibitor) and potassium binder. All patients had been taking iron sulfate for an average of 32.8 months (13-66 months).
Four patients underwent both upper and lower endoscopy, and the remaining two underwent upper endoscopy or colonoscopy. Numerous brown spots were observed on the mucosa of terminal ileum in 3 patients, duodenum in 1 patient, jejunum in 1 patient, and both duodenum and terminal ileum in 1 patient (Fig. 1). In one case with brown spots in jejunum, the patient had undergone subtotal gastrectomy with Billroth II reconstruction due to early gastric cancer.
Biopsies were taken during endoscopy and specimens were stained with hematoxylin and eosin and also stained for iron. Histology revealed pigment deposition within macrophages of the lamina propria and a positive Prussian blue stain indicated hemosiderin deposition (Fig. 2).
Small bowel pseudomelanosis is a finding that is rarely encountered during endoscopy and its etiology is still not fully understood (3, 5). Whereas melanosis coli is relatively common condition occurring in more than 70% of persons who use anthraquinone containing laxatives, pseudomelanosis of the small bowel is a very rare condition detected during endoscopy. Although examination of the small bowel has been facilitated with the introduction of capsule endoscopy (CE) or double balloon endoscopy (DBE), pseudomelanosis of the small bowel is still a rare finding. A Portuguese center with experience of more than 600 CE and 100 DBE reported only two cases of small bowel pseudomelanosis (2); one case showed pigmentation in the entire small bowel and the other case in the ileum (6). In Korea, pseudomelanosis duodeni or ilei has been reported sporadically in 7 cases in patients taking oral iron (7-9). Interestingly, 6 cases of pseudomelanosis ilei associated with charcoal ingestion have been reported only in Korea (10-14).
In our case series, all patients had been taking oral iron at the time of upper or lower endoscopy. Of the 6 cases, 3 had undergone upper and lower endoscopy before initiating iron therapy, which revealed normal mucosa without pigmentation on duodenum and terminal ileum. Therefore, the cause of pseudomelanosis of small bowel in these patients could be attributed to oral iron therapy. Apart from oral iron therapy, other possible causes of small bowel pseudomelanosis include chronic kidney disease, hypertension, and certain medications like antihypertensive drugs (3, 15). It has been hypothesized that coupling of absorbed iron with a sulfur moiety from antihypertensive medications could be the underlying mechanism for pigment accumulation in macrophages of small bowel mucosa (2). Fernando (16) demonstrated that absorbed iron coupled with sulfur in pseudomelanosis duodeni, using electron probe X-ray analysis of epithelial cells and macrophages at different levels of the intestine. He proposed that this coupling results in impaired iron transport that leads to the accumulation of ferrous sulfates. As for the source of sulfur, antihypertensive medications such as furosemide and hydrochlorothiazide which contain sulfur moiety have been implicated (17, 18).
Typically, the biopsy specimen shows dark brown to black granular deposits in the lamina propria of the tips of intestinal villi, and the deposits often contain iron as hemosiderin in ferric or ferrous form (19). A recent retrospective study showed that pigment-laden macrophages were sometimes observed even when it was not apparent during endoscopy (3). In another case of pseudomelanosis in which there were pigmentations from stomach to jejunum, review of previous histopathology slides from antral ulcer two years earlier revealed granular pigment in the lamina propria with positive stain for iron; however, pigmentation was not evident endoscopically at that time (20).
It could be postulated that duration of iron therapy is associated with the development of pseudomelanosis in the small bowel and determines its extent, but this association could not be validated possibly due to the small sample size. Although the causes of pseudomelanosis of the small bowel and its underlying mechanism are still unknown, our case series with a relatively large number of patients suggests that there could be a likely association between pseudomelanosis of the small bowel and chronic oral iron therapy.
Figures and Tables
Fig. 1
Endoscopic findings of cases. Endoscopic view of duodenum (case No. 1) (A), jejunum (case No. 2) (B), ileum (C) and duodenum (D) (case No.3) and ileum (E) and ileocecal valve (F) (case No. 6) shows a brown pigmentation with a speckled, continuous pattern.
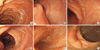
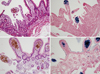
Fig. 2
Histopathologic findings of cases using biopsy specimens. Histological examination of duodenal (A) and ileal (B) mucosa showing brown pigment deposition within macrophages in the lamina propria (stain, × 200) and (C, D) intense pigmentation revealing iron deposition as hemosiderin (blue coloration; Perl's Prussian blue stain, × 200).
References
1. Freeman HJ. "Melanosis" in the small and large intestine. World J Gastroenterol. 2008. 14:4296–4299.
2. Almeida N, Figueiredo P, Lopes S, Freire P, Sousa V, Lérias C, Gouveia H, Leitão MC. Small bowel pseudomelanosis and oral iron therapy. Dig Endosc. 2009. 21:128–130.
3. Giusto D, Jakate S. Pseudomelanosis duodeni: associated with multiple clinical conditions and unpredictable iron stainability - a case series. Endoscopy. 2008. 40:165–167.
4. Kibria R, Barde CJ. Pseudomelanosis of the stomach. Endoscopy. 2010. 42:E60.
5. Kibria R, Ali SA, Akram S. Pseudomelanosis ilei associated with ingestion of oral iron therapy. Endoscopy. 2010. 42:E243–E244.
6. Wald A. Feldman M, Freidman LS, Brandt LJ, editors. Other disease of the colon and rectum. Sleisenger and Fordtran's gastrointestinal and liver disease. 2010. 9th ed. Philadelphia: Sounders Elsevier;2242–2255.
7. Lee KH, Lee TH, Shim YS, Choi JH, Chung IK, Park SH, Kim SJ, Oh MH. Two cases of pseudomelanosis duodeni associated with systemic disease and oral iron supplementation. Korean J Gastrointest Endosc. 2009. 39:374–378.
8. Cha JM, Lee JI, Joo KR, Jung SW, Shin HP. Melanosis ilei associated with chronic ingestion of oral iron. Gut Liver. 2009. 3:315–317.
9. Lee KW, Jeong WJ, Lee JH, Kang JW, Jang KH, Han KH, Kang GH, Cheon GJ. Simultaneous melanosis duodeni and melanosis ilei in a patient taking oral iron. Korean J Gastrointest Endosc. 2010. 41:308–311.
10. Kim J, Hwang JK, Choi WS, Lee BJ, Park JJ, Kim JS, Bak YT, Kim I. Pseudomelanosis ilei associated with ingestion of charcoal: case report and review of literature. Dig Endosc. 2010. 22:56–58.
11. Kim GM, Jun EJ, Kim YC, Park JM, Hong SI, Cheung DY, Kim JI, Lee YS. Melanosis ilei induced by prolonged charcoal ingestion. J Korean Surg Soc. 2011. 81:66–69.
12. Kim SY, Koo JS, Hynun JJ, Jung SW, Choung RS, Yim HJ, Lee SW, Choi JH. Charcoal-induced pseudomelanosis ilei. Endoscopy. 2011. 43:E380.
13. Bae MS, Bae RC, Shin MC, Park KH, Cho SJ. Charcol-induced anthracosis of the terminal ileum. Korean J Med. 2009. 76:490–493.
14. Kim MS, Park YB, Ha BW, Cheung DY, Kim JI, Cho SH, Park SH, Kim JK. Two cases of melanosis ilei developed after long-standing charcoal ingestion. Korean J Gastrointest Endosc. 2008. 36:36–39.
15. Yun L. Education and imaging: gastrointestinal: pseudomelanosis duodeni. J Gastroenterol Hepatol. 2010. 25:427.
16. Fernando SS. Pseudomelanosis duodeni: a case report with electronprobe X-ray analysis. Pathology. 1990. 22:169–172.
17. Leong S. Pseudomelanosis duodeni and the controversial pigment: a clinical study of 4 cases. Ann Acad Med Singapore. 1992. 21:394–398.
18. Pueblitz S, Squires RH, Timmons CF. Pseudomelanosis duodeni in an adolescent male: case report and review of the literature. Pediatr Pathol Lab Med. 1997. 17:115–123.
19. Krier K, Henderson JB, Bocklage T, Rajput A. Duodenal pseudomelanosis. Surgery. 2012. 151:129–130.
20. Weinstock LB, Katzman D, Wang HL. Pseudomelanosis of stomach, duodenum, and jejunum. Gastrointest Endosc. 2003. 58:578.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download