Abstract
The incidence of overall cancer has increased over time. The incidence of top-ranking cancers has changed in the 1990s and the 2000s. However, few studies have evaluated the trends in metastatic skin cancers during this period. We evaluated the recent trends in incidence, peak age and location of metastatic skin cancers from 1991 to 2010. This 20-yr survey was divided into two decades to determine the trends by comparing the statistics. Out of 694,466 outpatients (1991-2010), 174 (0.025%) were diagnosed with metastatic skin cancer. The incidence of metastatic skin cancer increased significantly from 20.64 per 100,000 outpatients in the 1990s to 28.70 per 100,000 outpatients in the 2000s (P = 0.030). The peak age of skin metastasis shifted from the 40s to the 50s in women, and from the 50s to the 60s in men. The percentage of metastatic skin cancers originating from intra-abdominal organs increased from 10% in the 1990s to 23.1% in the 2000s (P = 0.027). The percentage of metastatic skin cancers located on the abdomen increased from 7.1% in the 1990s to 15.4% in the 2000s (P = 0.011). The higher proportion of metastatic skin cancers located on the abdomen may be related to the increase in skin metastases from intra-abdominal organs.
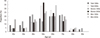
The incidence of cancer has increased over time in Korea and worldwide. The overall incidence of cancer was reported to be 210.5 per 100,000 in 1999 (1). This incidence increased to 254.5 per 100,000 in 2007 (1). The increases in the rates of each cancer are variable, and the top-ranking cancers in the 1990s and the 2000s are different. For example, the incidences of thyroid cancer and colon cancer has increased dramatically, while the incidence of cervical cancer has decreased (1) (Fig. 1).
Since the top-ranking cancers in the 1990s and the 2000s are different, the incidence of skin metastasis is also expected to change. However, from our clinical experience, it is unlikely that the incidence of metastatic skin cancer in the 1990s and the 2000s is proportional to that of primary cancers. To date, there have been few reports analyzing the trends of metastatic skin cancer worldwide (2, 3). In Korea, previous studies of metastatic skin cancer have been limited to common cancers such as lung or breast cancer (4-10). Furthermore, there have been no studies analyzing the recent trends of metastatic skin cancer in decades. In this study, we evaluated recent trends of metastatic skin cancer from 1991 to 2010 in terms of incidence, peak age and location.
We performed a retrospective study of metastatic skin cancers diagnosed in the Department of Dermatology of eight affiliated hospitals of the Catholic University of Korea from July 1991 to June 2010. Three of the hospitals are located in Seoul, four in Gyeonggi-do Province (Uijeongbu, Bucheon, Incheon and Suwon), and one in Daejeon. Out of 694,466 outpatients (1991-2010), 174 (0.025%) were diagnosed with metastatic skin cancer. The diagnoses were confirmed by histopathological reviews.
We divided the patients into two groups, one group of patients who were diagnosed with metastatic skin cancer in the 1990s and the other group diagnosed in the 2000s. There were 70 patients with metastatic skin cancer from the sample taken in the 1990s and 104 patients from the sample taken in the 2000s (Fig. 2). The primary outcome measures were the incidence rate of metastatic skin cancer based on the dermatology outpatient during the 20-yr period and the difference in incidence over the two decades (1991-2000 vs 2001-2010). We calculated the incidence rate by dividing the number of patients with metastatic skin cancer into the total outpatient number for 10 yr. To evaluate the increasing or decreasing patterns of incidence, we used Poisson regression. In addition, we evaluated the average age and gender distribution of patients, the origin and the location of metastatic skin cancers from the patient's medical records. Chi-square tests were used to compare these data over the two consecutive periods. The change in the frequency of the primary cancer was assessed by Fisher exact test or chi-square test. The significance level was P < 0.05. Statistical analysis was performed using SPSS for Windows version 12.00 (SPSS Inc., Chicago, IL, USA).
The incidence of metastatic skin cancer in the 1990s was 20.64 per 100,000, and it increased significantly to 28.70 per 100,000 in the 2000s (P = 0.030) (Fig. 2A). Furthermore, there was a significant increase in the annual incidence of metastatic skin cancers from 1991 to 2010 (P = 0.001) (Fig. 2B).
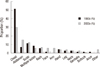
During the total study period, females outnumbered males in incidence among the patient samples. The male to female ratio in the 1990s and the 2000s was 1:1.8 and 1:1.76, respectively. There was no significant difference in the male to female ratio between the 1990s and 2000s. There was a significant difference in the age distribution of the patients with metastatic skin cancer between the two groups (P < 0.001). In the 1990s, the percentages of patients with metastatic skin cancer were 8.6%, 8.6%, 20%, 17.1%, 20%, 21.4%, 1.4%, and 2.9% for patients in their 20s, 30s, 40s, 50s, 60s, 70s, 80s, and 90s, respectively. In the 2000s, the percentages were 2.9%, 4.8%, 12.5%, 33.7%, 27.9%, 14.4%, 13.8%, and 0%, respectively. There was a significant difference in the proportion of patients in their 40s and 50s between the 1990s and the 2000s (40s: P < 0.001, 50s: P = 0.004). The peak age of men in the two groups shifted from the 50s to the 60s, and that of women from the 40s to the 50s (Table 1) (Fig. 3).
The origins of the top three primary cancers (breast, lung, and stomach, in decreasing order) were identical in the two groups (Fig. 4). Of 174 patients with metastatic skin cancer, the percentage of patients with breast cancer slightly decreased from 40% to 37.5%, whereas that of lung cancer and stomach cancer slightly increased from 18.6% to 19.2%, and from 7.2% to 8.7%, respectively. Moreover, the majority of these cancers did not show a significant difference between the study periods. Likewise, none of the intra-abdominal cancers showed a significant difference between these periods (Table 2).
From our clinical experience the proportion of metastatic skin cancer located on the abdomen seems to have increased over time. As such, we next examined the incidence of metastatic skin cancer originating from the intra-abdominal organs. The incidence of overall metastatic skin cancers originating from the intra-abdominal organs significantly increased from 10% to 23.1% (P = 0.027). Moreover, the incidence of metastatic skin cancers originating from the intra-abdominal organs, with the exception of stomach cancer, quadrupled from 2.8% to 14.4% (Fig. 4B).
There was a significant difference in the anatomical location of metastatic skin cancers between the two groups (P = 0.011). The most common location was the chest in both groups, but the percentage of metastatic skin cancers located on the chest decreased over time. On the other hand, the incidence of metastatic skin cancers located on the abdomen increased from 7.1% in the 1990s to 15.4% in the 2000s (Table 3) (Fig. 5).
This is the first study investigating the trends in metastatic skin cancers in Korea. There have been few studies evaluating the incidence of metastatic skin cancer among dermatology outpatients. In prior studies, the incidence of metastatic skin cancer was estimated based on patients with internal malignancies, not among dermatology outpatients (2, 11-15). Since the purpose of previous studies was to investigate the characteristics of metastatic skin cancer from each internal organ cancer, the incidence of metastatic skin cancer was calculated from patients with internal malignancies. Therefore, the incidence of metastatic skin cancer varies according to the primary cancer in the previous studies (2, 11-15). The purpose of the present study is to compare the trends of metastatic skin cancers between the 1990s and the 2000s. When the incidence of metastatic skin cancer is calculated from dermatology outpatients, we can identify changes in incidence, which is more suitable for our purpose.
The incidence of metastatic skin cancer increased from the 1990s to the 2000s. The Poisson regression analysis demonstrated that the incidence of metastatic skin cancers has been increasing each year, and it is increasing at a rapid rate in recent years. Since the Poisson regression analysis revealed an increasing tendency, it is assumed that the incidence of metastatic skin cancers may increase in the near future (Fig. 2B). Similar to our results, Nashan et al. (11) reported that the incidence of cutaneous metastases from the patients with internal organ malignancies increased dramatically from 2.7% in 1969 to 4.5% in 1993, and to 10% in 2009 (11, 12). They also suggested that the incidence of metastatic skin cancers has been increasing due to rising cancer rates and longer survival rates (11).
The important factors that influence skin metastasis of primary cancer may include the incidence and the rate of cutaneous metastasis from primary cancer. The incidence of metastatic skin cancers was not always proportional to that of each primary cancer (Fig. 1, 4). The rates of cutaneous metastasis vary; for example, the rates are 2.42% in breast cancer, 1.78% in lung cancer, 0.8% in colon cancer and stomach cancer, and 0.2% in thyroid cancer (2). When analyzed together, the incidence of metastatic skin cancers is proportional to that of primary cancer, multiplied by the rate of cutaneous metastasis. For example, the incidence of lung, stomach, and colon cancer in 2009 was 39.6, 59.9, and 50.3, respectively (16). When the incidence of each cancer was multiplied by the rate of cutaneous metastasis, the incidence was 70.5, 47.9, and 40.2, which is in line with the rates in Fig. 4. Therefore, to estimate the incidence of metastatic skin cancer, the rate of cutaneous metastasis and the incidence of primary cancer are helpful. There have been few studies that investigate the rates of cutaneous metastasis from those of primary cancer, but more studies will be necessary.
In this study, metastatic skin cancer from the stomach was the third leading origin of skin metastases, in contrast to prior studies that reported colon cancer as the third leading origin (15, 17-19). Stomach cancer is the most frequent cancer in Koreans, unlike other populations (1, 17-19). The rate of cutaneous metastasis of the stomach and colon are same (2). Therefore, the incidence of primary cancer determines the incidence of metastatic skin cancer in this case.
The peak age of the patients with metastatic skin cancer increased during the two decades examined in this study. The peak age of patients with breast cancer was in the 40s and did not change from 1998 to 2012 (20, 21). In contrast, the peak age of patients with cutaneous metastasis in breast cancer shifted from the 40s to the 50s in this study. The early detection of primary cancer and the development of new cancer treatment may extend survival and metastasis-free period. Statistically, the 5-yr relative survival rates were higher for most major cancers in patients diagnosed during 2005-2009 compared with those diagnosed between 1993-1995 (22). Likewise, targeted therapy and tailored chemotherapy for advanced non-small cell lung cancer such as erlotinib and bevacizumab lengthen the survival rates of lung cancer patients (23, 24).
The location of primary cancers is another important factor influencing skin metastasis. It is believed that the skin metastasis from internal organ cancers follow definite patterns of distribution (25, 26). In general, skin metastases tend to most frequently occur in the vicinity of the primary cancer. Breast and lung cancers frequently metastasize to the chest wall, whereas cancers of the bowel, ovary, and bladder most often metastasize to the abdomen (15). Direct invasion is thought to be one of the most important pathways (3). Breast cancer and lung cancer have greater accessibility to the skin than other primary cancers. Intra-abdominal cancers may be less likely to metastasize to the skin since the peritoneum and thick fat layer can block metastasis. Therefore, the skin metastases of breast cancer and lung cancer have been more common than those of intra-abdominal organ cancer in the past two decades, although the incidence of primary cancer from an intra-abdominal organ is much higher than that from the breast cancer and lung cancer.
Over the past two decades, the most common site of cutaneous metastasis has been the chest because the top-ranking primary cancers have been breast, lung and stomach cancer, and these rankings did not change during this period. In contrast, as the proportion of intra-abdominal cancer, especially stomach cancer and colon cancer, has increased significantly, the proportion of metastases in the abdomen has increased. Advances in adjuvant therapy and surgical management are thought to increase the survival of the stomach cancer and colorectal cancer patients (27). It showed the greatest improvements in the 5-yr relative survival rate between 1993 and 2005 in Korea (28). As a result, surviving stomach or colorectal cancer patients with skin metastasis are increasing. The most common clinical features of metastatic skin cancer from the intra-abdominal organ are pedunculated or fixed nodules and masses (3, 13, 15). Cutaneous metastases may represent the first evidence of malignancy (13). Therefore, if there are nodules or masses with irregular borders or fixed tendency in the abdomen, clinicians should be concerned about the possibility of metastasis and must confirm them with biopsy.
The limitation of this study is that the number of total patients with skin metastasis was small. Since the data was collected from eight affiliated hospitals, the findings may not be generalized to all patients with metastatic skin cancers in Korea.
In conclusion, it is suggested that the recent increase in intra-abdominal origin cancers has led to the relative increase in the proportion of metastatic skin cancer to the abdomen.
Figures and Tables
Fig. 2
The number of metastatic skin cancer patients during 1991-2010 (A) The number of metastatic skin cancer patients and the comparison of the incidence of metastatic skin cancers between the 1990s and the 2000s in the total dermatologic outpatient population. (B) The number of metastatic skin cancer patients by year with increasing slope with exponential pattern (P = 0.001 by Poisson regression).

Fig. 3
The age and sex distrubution of the metastatic skin cancer patients in the 1990s and the 2000s.
References
1. Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010. 25:1113–1121.
2. Hu SC, Chen GS, Wu CS, Chai CY, Chen WT, Lan CC. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009. 60:379–387.
3. Hu SC, Chen GS, Lu YW, Wu CS, Lan CC. Cutaneous metastases from different internal malignancies: a clinical and prognostic appraisal. J Eur Acad Dermatol Venereol. 2008. 22:735–740.
4. Chang SE, Choi JC, Choi JH, Sung KJ, Moon KC, Koh JK. Prevalence and clinicopathological features of cutaneous metastasis from lung cancer in Korea. Korean J Dermatol. 2001. 39:660–665.
5. Roh KH, Chang SE, Lee MW, Choi JH, Moon KC, Koh JK. Clinicopathological study of cutaneous involvement from breast cancer. Korean J Dermatol. 2003. 41:1318–1322.
6. Kim DH, Lee JD, Cho SH, Oh SJ. Clinical study of dermatologic disorders in patients with breast cancer. Korean J Dermatol. 2004. 42:1285–1293.
7. Yi JH, Moon WS, Yun SK, Kim HU, Ihm CW. Clinicopathological study on metastatic skin cancer. Korean J Dermatol. 2006. 44:567–573.
8. Park SJ, Lee DY, Lee ES. Clinicopathological study of 43 cases of metastatic skin cancer. Korean J Dermatol. 2002. 40:639–645.
9. Lee CN, You CE, Park HJ, Park CJ, Cho SH, Lee JY, Byun DG, Kim JW, Kim SY, Kim HO, et al. Metastatic cancer of the skin: clinical and histopathologic study. Korean J Dermatol. 2002. 40:1212–1218.
10. Kim SH, Jeon YS, Sim HJ, Suh KS, Kim ST. Clinicopathologic findings of metastatic skin cancer. Korean J Dermatol. 2004. 42:300–308.
11. Nashan D, Müller ML, Braun-Falco M, Reichenberger S, Szeimies RM, Bruckner-Tuderman L. Cutaneous metastases of visceral tumors: a review. J Cancer Res Clin Oncol. 2009. 135:1–14.
12. Poole S, Fenske NA. Cutaneous markers of internal malignancy: I. malignant involvement of the skin and the genodermatoses. J Am Acad Dermatol. 1993. 28:1–13.
13. Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995. 33:161–182.
14. Brodland DG, Zitelli JA. Mechanisms of metastasis. J Am Acad Dermatol. 1992. 27:1–8.
15. Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993. 29:228–236.
16. National Statistical Office. Headquarters of Korea Central Cancer Registry. Ministry of Health and Welfare. Table: cancer incidence and incidence rate. accessed on 5 Jun 2013. Available at http://www.index.go.kr/egams/stts/jsp/potal/stts/PO_STTS_IdxMain.jsp?idx_cd=2770.
17. Chiang CJ, Chen YC, Chen CJ, You SL, Lai MS. Taiwan Cancer Registry Task Force. Cancer trends in Taiwan. Jpn J Clin Oncol. 2010. 40:897–904.
18. Fitzgerald TL, Bradley CJ, Dahman B, Zervos EE. Gastrointestinal malignancies: when does race matter? J Am Coll Surg. 2009. 209:645–652.
19. Ali R, Barnes I, Kan SW, Beral V. Cancer incidence in British Indians and British whites in Leicester, 2001-2006. Br J Cancer. 2010. 103:143–148.
20. The Korean Breast Cancer Society. Clinical characteristics of Korean breast cancer patients in 1998: the Korean Breast Cancer Society. J Korean Med Sci. 2000. 15:569–579.
21. Jung KW, Park S, Won YJ, Kong HJ, Lee JY, Seo HG, Lee JS. Prediction of cancer incidence and mortality in Korea, 2012. Cancer Res Treat. 2012. 44:25–31.
22. Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012. 44:11–24.
23. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005. 353:123–132.
24. Eskens FA, Sleijfer S. The use of bevacizumab in colorectal, lung, breast, renal and ovarian cancer: where does it fit? Eur J Cancer. 2008. 44:2350–2356.
25. Rolz-Cruz G, Kim CC. Tumor invasion of the skin. Dermatol Clin. 2008. 26:89–102.
26. Tschen EH, Apisarnthanarax P. Inflammatory metastatic carcinoma of the breast. Arch Dermatol. 1981. 117:120–121.
27. Gondos A, Bray F, Hakulinen T, Brenner H. EUNICE Survival Working Group. Trends in cancer survival in 11 European populations from 1990 to 2009: a model-based analysis. Ann Oncol. 2009. 20:564–573.
28. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013. 45:1–14.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download