Abstract
Noting the increasing public attention on healthcare, Korean society has shown greater attention to the significance of the health technology (HT) development. In order to promote HT competitiveness, the role of research-based hospitals (RBHs), in producing new ideas as well as utilizing final outcomes, has grown increasingly significant. Despite high quality healthcare professionals, state-of-the-art equipment, and well-developed information technology, few hospitals in Korea are successful leaders in HT development. In order to understand HT research and development (R&D) programs in Korea as well as hospital-based R&D investment performance, this paper has analyzed a recent three-year R&D investment of the Korean government. In addition, a survey on how to promote RBHs in Korea has been proceeded through adopting the Delphi method. Several model cases of RBHs abroad have also been studied to understand key success factors in formulating a development model of RBHs in Korea. This paper proposes suggestions for the promotion of RBHs in Korea: systematic reform related to the hospitals, reinforcement of the infrastructure of the hospitals, empowering human resources and policy framework to support the hospitals.
Hospitals are the source of technology development for the health sector as they provide ideas based on clinical experiences and as the end-users of the developed technologies; they are at the center of the value chain for Health Technology (HT) industry development (1). Therefore, it is highly requested for competent hospitals to connect industrialization with medical services and to develop HT (2). Note, however, that active stimulation of medical personnel and hospital research is very important in view of the current income structure of the hospitals, which depends largely upon medical service provision. The improvement of the hospitals' situation to encourage research by physicians is considered a matter of urgency, with regard to improving their own financial stability and further development.
This paper seeks to search for a method to shape research-based hospitals as a framework tool for Korea's research and development (R&D) capacity, a key factor in the health industry's development. In such a context, this paper aims to draw a model of research-based hospitals in Korea and to also propose suggestions for hospitals, industry, and policy-makers to accomplish such model. This paper intends to encourage the investment in the R&D of health and medical services by positioning the hospitals at the center of HT R&D within close coordination and strong connection between beds and benches. This paper tries to search for alternative suggestions concerning systems, human resource training and management structure, and the policy environment of many hospitals, in order to build a Korean-style research-based hospital model. This type of hospital model would allow many clinical service provision dominant hospitals with potentiality of HT R&D to possibly transfer to balanced hospitals in research and medical service provision.
This paper targets approximately 30 experts including researchers at hospitals who have actively participated in R&D, CEOs of hospitals, and technology transfer organization directors to study whether Korea's hospitals have the capacity for HT R&D and play a pivotal role. For the questionnaire survey and analysis, a qualitative analysis method, applying the Delphi technique (3), is used in view of the small size of samples.
To examine the recent research achievements of major research hospitals, this paper analyzes the amount of R&D investment in the health sector supported by the Korean Ministry of Health and Welfare (MOHW) by classifying each hospital grant for the past three years (MOHW 2008; MOHW 2009), whose data was available and categorized based on the amount of R&D fund spent by each hospitals. Although a head of a research team of a single project posts the statistics of the total research expenses to his/her institution in general for large-scale projects, this paper examines which hospitals have executed research investment per detailed project for the analysis of the actual research investment amount.
Along with the analysis above, this study also investigated the papers, patents and technology transfer, and royalty amount produced by the recent MOHW research subsidies per hospital. Through this, the amount of research investment made for one paper and research investment made per patent were examined to measure each hospital's R&D efficiency. Note, however, that this study analyzed R&D achievements such as papers and patents in a quantitative method. Qualitative analysis such as the impact factor of an individual paper or a patent's industrial values has been excluded.
To analyze Korean hospitals' current research capacity, this study examines the status of research grants and performances (particularly examines papers, product commercialization cases, technology transfer cases, etc.) supported by MOHW for hospitals including medical colleges, colleges of dentistry, and colleges of Korean traditional medicine from MOHW's R&D budget for the past 5 yr, 2005-2009 (Table 1). The hospital-based R&D investment ratio among the total R&D fund of MOHW showed an upward trend from 45% in 2005 to 67% in 2009. The number of supported projects had risen as well, with 486 projects or 60% of the total projects as of 2009 (4). Hospitals and medical colleges located in Seoul and the Metropolitan Seoul Area received about 80% of total R&D grants for hospitals. This data showed the concentration of HT R&D grants by MOHW in the greater Seoul region.
Looking into the performances of hospital-based R&D funded by MOHW, the number of papers supported by R&D programs were higher than those not funded. However, the number of patents and product commercialization were comparatively low. Most probably, the reason for this less achievement of reaching patent attainment and commercialization was that these R&D projects aiming for commercialization mostly support pharmaceuticals or medical equipment companies. For patents and technology transfers, however, the interests of hospital researchers or hospital-based technology transfer organizations (TTOs) were estimated to be insufficient so far. The fact that paper achievements of hospitals are far more excellent than non-hospital institutions implies that the R&D potential of hospitals has already matured to some degree. Moreover, in the future, if proper support is offered for the insufficient parts, hospital R&D potential can be upgraded rapidly into an innovative level in order to boost HT-based industries in Korea.
To more specifically examine the status of HT R&D programs in hospitals in Korea, this study classifies R&D grants from MOHW for the past three years from 2007 to 2009 when data was available based on the actual amount spent by hospitals. Likewise, the produced papers, patents, product commercialization, and technology transfer cases have been investigated. For the past three years, a total of 45 hospitals carried out at least one project, subsidized by MOHW. The difference in research subsidies was a whopping 2.5 times that figures with KRW 63.8 billion as the largest subsidy given to a hospital for the past three years followed by KRW 26.0 billion. Five medical institutions received more than KRW 20 billion in subsidies for the past three years. At least 23 medical institutions were located in provincial areas. Of these provincial hospitals, the largest subsidy was KRW 8 billion over the past three years.
Based upon the collective opinion revealed by the experts' surveys, several factors were recognized as crucial for an efficient and effective research-based hospital. Even though there are significant differences in terms of the financial environment, national health security system, educational structure and value and framework of the national economy, the case studies on advanced RBHs abroad also show that there should be universal key success factors that can be benchmarked into the Korean context. As a whole, the most significant factor is the hospital system. The system covers not only the operational management of the hospital but also the incentive system for all staff members, the structure of human resources management, the criteria of resources allocation and the networking capability of industrial and academic societies. The entire hospital system should be oriented for research and medical service provision equivalently.
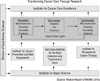
The second factor is the goal of the hospital, which is to be research-oriented. As shown in the case study on the MD Anderson Cancer Center (MDACC), the priority of the hospital's mission is research and development (5). This orientation is encouraged by an incentive system for staff members. Every employee is given a predictable incentive of income and status based upon research performance as well as provision of medical services. Even the physicians' income and job security are determined both by research and performance of medical services. In MDACC, physicians with R&D grants are responsible for fewer patients while those without grants will be responsible for more medical services (5). In other words, there is no negative incentive for employees to devote themselves to research activities or reduce their amount of medical service (Fig. 1).
This research-oriented model also has been supported by the consensus of all the stakeholders in the hospital. From the CEO to medical doctors and technicians, there is a firm belief that their hospital should be developed through research and development as well as providing first-rate medical services, as shown in the case studies of MDACC and Johns Hopkins. Especially strong commitment and leadership of CEOs are present in all of the advanced research-oriented hospitals including MDACC, Johns Hopkins and Tokyo Women's University Hospital and Waseda Joint Institution for Biomedical Science (TWIns).
Another factor is a research-friendly arrangement of infrastructure. For example, inadequate space and equipment are frequently mentioned in the survey as an important barrier to overcome for an RBH. In the case study of the TWIns, a 22,000 m2 building is solely dedicated to this research institute (6). Also, this building has its own GMP facilities along with other relevant laboratories and equipments for research and development. Another crucial factor is human resources. The case study shows that all successful models have a proper combination of the following human resources: R&D program coordinators, technicians, research nurses, financial managers, intellectual property specialists, government relations experts, legal affairs managers, secretaries for R&D, and research-oriented scientists and medical doctors.
In addition, adequate investment of R&D funding is crucial in the achievement of a research-based hospital. For example, MD Anderson spent $488,654,827 in 2008, which was more than double the amount of total R&D funding from MOHW Korea in the same fiscal year. The top-ranked hospitals in terms of receiving R&D grant from MOHW in 2009 received 27,141 million Won, and the average MOHW R&D grant to the top 5 hospitals was 6,140 million Won during 2007 to 2009. To maintain a hospital, R&D is a high-risk investment, considering that hospitals have an extremely low-risk investment in the provision of medical services. Thus, hospitals cannot invest in research activities if there is insufficient R&D grant money given by the government. Hospitals cannot bear uncertain high-risk investment in R&D, which should be sponsored by a government-backed risk pool.
The final factor is successful team collaboration, including interdisciplinary and multidisciplinary approaches. The Cancer Prevention and Research Institute of Texas (CIPRIT) requires multidisciplinary collaboration as a prerequisite condition for application. The hospital of the University of California at San Francisco established an R&D building for hosting multidisciplinary research teams. Also, TWIns itself is an interdisciplinary R&D entity between Tokyo Women's Medical University and Waseda University, which does not have a medical college. TWIns is a joint team of a medical school and science department of two schools for HT R&D. This kind of team collaboration also requires experts of integrating diverse sectors. These experts are called integrators, translators, or coordinators, many of whom are medical doctor-scientists with deep understanding in pathology, biochemistry, and physiology.
Strong leadership for R&D, a reasonable incentive system for researchers, adequate resource allocation for R&D, capable human resources, appropriate R&D funds, and well-organized team collaboration are key success factors identified through the survey and case studies. Although there may be variations between hospitals, those key success factors can also be applied in Korea to establish successful RBHs.
Some hospitals in Korea have high potentiality for HT R&D. With top-quality human resources including medical experts, state-of-the-art medical equipments in quantity, large numbers of patients groups, and digitalized information system, these hospitals could turn into research and development leaders in HT improvement. However, many experts replied that the Korean hospitals are not taking a leadership role in R&D despite their high potentiality of HT R&D innovation. In this regard, there are key factors to stimulate hospital-based research and to nurture hospitals to become the hub of HT R&D. Those key success factors can be identified as "tipping points", critically affecting and changing hospitals in the direction of R&D.
For the discussion on policy initiatives, it is required to define the concept of the RBH. The task force team on the RBH at the Ministry of Health and Welfare defines it as "a world-class RBH which leads the development of health industry through research and business based development (R&BD) on the state-of-the-art health technology, based upon accumulated knowledge through clinical experiences within the hospital" (7). This concept of a RBH has several elements: 1) world-class hospital, 2) leader of the health industry, 3) core of R&BD in HT, and 4) user of accumulated knowledge from clinical experiences.
In order to effectively establish RBHs in Korea, the systematic reform of the hospital is the most significant factor. A crucial point is that all staff members seriously regard R&D as a priority and that non-R&D staffs as well as researchers fully understand the value and the necessity of HT R&D in the hospital. This goal of the RBH model cannot be reached without multi-dimensional collaboration of the hospitals, the government, health industries, universities and research institutions. In this study, a set of policy initiatives, required for the strategic reform of the hospitals, is discussed. They are composed of systematic reform related to hospitals, reinforcement of infrastructure and human resources, and revision of government policies.
Leadership of a hospital is a critical factor in determining the management and performance of the hospital. However, CEOs of hospitals in Korea are usually evaluated on a comparatively short-term basis which is reported to affect their management direction of CEOs to focus on short-term outcomes especially in financial performance. In this regard, a policy is required to encourage selected hospitals to adopt a long-term basis evaluation system along with a revision of evaluation factors including bigger portions for research activities and strategic management of hospitals.
While strong leadership is essential, voluntary cooperation from all hospital employees is also critical for a successful RBH. A major problem is that the employees cannot find enough time and resources for R&D within the hospital. Furthermore, physicians are not motivated to devote themselves to R&D mainly due to a lack of incentives, which is oriented by the instant and reliable income of the hospital. Because R&D is a long-term and unpredictable investment, researchers are not appropriately motivated under the current incentive system, which significantly affects researchers' income, organizational status and job security. On the other hand, it is inferred that many researchers see hence a burden medical treatment assignments and R&D activity as an extra work.
In order to guarantee continuous cooperation of all members of the hospitals for R&D, a strategic management system should be predictable and persistent, instead of counting on personal-based commitment. Regarding a salary arrangement system, the hospitals should apply a policy of equivalent treatment between profit from R&D and medical services. Thus, a physician can expect the same income incentive from R&D activities. Also, the other employees of the hospital may consider the R&D program as one of the main activities of the hospital under this policy.
In addition, a performance evaluation for employees should not only include medical service, but also outcomes of R&D. Based on this comprehensive evaluation system, a researcher may have incentives for promotion and tenure-track according to research performance. Economic incentives along with non-economic favors can encourage R&D activities of the hospitals, which is a primary factor in founding an RBH. Instead of relocating direct employees to research, this kind of indirect incentive policy may lead to becoming a research-oriented hospital.
One of the key success factors of a RBH is close team collaboration among related units of the hospitals. The multi-dimensional approach is essential because a competent R&D hospital not only needs the existence of specialized sub-units but also a framework for multi-disciplinary collaboration. To stimulate team collaboration, a hospital may impose incentives for multidisciplinary projects in an investment decision guideline. Also, a hospital should support this by providing regular meetings of diverse experts, financing multi-units training courses, and recruiting required staffs to aid the team approach.
Another cultural characteristic required for RBH is openness of the hospital system. Not only internal cooperation among sub-units of a hospital but also close collaboration with external resources, such as health industries and research institutions, are essential to RBHs. In Korea, the Seoul Asan Hospital works with a couple of bio-venture companies within its campus. In this setting, both hospital and the company enjoy mutual benefit through close collaboration between physicians and researchers of the two institutions.
Some of the hospital-based research projects may succeed in commercialization, which brings income to the hospitals. It is recommended to adopt an obligatory policy to re-invest a certain portion of the profit made from R&D outcome of the hospital, to ensure continuous investment in R&D. Also, it is meaningful to introduce this policy in the initial stage of RBH development, considering that the entire R&D fund of Korea is limited and the social recognition of hospital-based R&D is lacking.
It is essential to build a strong technology transfer organization (TTO) to support close and efficient communication between researchers of the hospitals and relevant companies. In-hospital researchers have knowledge, experience, and ideas for managing an R&D project; on the other hand, most of them require assistance from experts on how to apply their research outcomes into the industry. In addition, health industries and investment funds may not easily be able to identify R&D procedures in hospitals although they, as potential investors, are eager to discover appropriate items. Strong TTOs located in hospitals will contribute in bridging research and industry, which will result in building the concrete value chain of HT R&D.
As discussed, pertinent allocation of space and equipment for researchers is imperative for a successful RBH. A comprehensive and well-organized information system for all stakeholders of hospital-based R&D is recommended. For efficiency and effectiveness of R&D project management, an improved information system is necessary to provide all relevant information to researchers, assistants, and managers of the hospitals about real-time processes of research projects, potential resources such as government grants, non-government funds, and details on researchers.
Clinical trial research is increasingly important as the market size grows by 3% annually and globally. Reliable clinical trial research facilities must be established in order to ensure the implementation of successful research programs within hospitals. It will help the researchers of the hospitals conduct research more efficiently and health industry companies communicate successfully with researchers.
In an RBH, a dual-track career development system for physicians is recommended. In this system, a physician can choose his or her own major career track between research and medical service. Even though a physician can choose the research track, he or she is recommended to invest a certain portion of his or her time on medical service provision. Without clinical experience and knowledge, physicians cannot successfully carry out research projects because their main role is to direct and coordinate the whole project based upon clinical knowledge.
Training course exchanges are recommended to educate physicians and scientists. Exchanges between medical colleges and life science colleges will increase the understanding of each other, which will strengthen professional relationships and development of research projects of the hospitals. A career management program is required to encourage basic scientists and technicians to work in hospital-based research.
The most serious challenge is to advocate for the value of RBH in Korea. The value of health technology and industry should be recognized along with the role of RBH in HT R&D. In this regard, an accreditation of RBH can be developed as well as a social recognition system for renowned HT researchers. For systematic support of RBHs, legislation is required. It should prescribe the legal concept, elements, and authorization of diverse policy support for RBHs.
Deregulation is definitely required for fostering RBHs. Initially, the overhead cost allowed in government grants needs to be raised because hospitals will take a significant financial loss from investing in physicians for research activities instead of medical services. This policy will allow the government to share the burden of the economic loss of hospitals. In addition, more flexibility regarding the use of the R&D grant is required.
Another required deregulatory action concerns the National Health Insurance (NHI). If NHI allows temporary non-reimbursable coverage for drugs, medical equipment, or techniques newly developed by an RBH, the health industry will improve significantly through hospital-based researches. Since expensive costs are required for researchers to apply outcomes of R&D to the clinical practice, this kind of deregulation will help to nurture RBHs in Korea effectively.
Moreover, the restriction on the establishment of venture companies by an RBH for the commercialization of R&D outcomes is necessary. Under the current regulation, most hospitals that are non-for-profit entities cannot establish venture companies, which may be a barrier in the commercialization of R&D outcomes. Despite expected negative effects, carefully developed deregulation can boost R&BD in hospitals.
If a World-Class Research-based Hospital (WCRBH) project can be initiated by the government, the hospitals with R&D capacity will take more concrete actions towards RBH establishment. As shown in the case studies, a huge amount of funds have been invested to advanced research-oriented hospitals in the USA and Japan. Sharply increasing the investment in HT R&D by launching the "WCRBH" project, hospital-based HT research can be the core framework for HT R&D.
With regard to coordination of the government investment on HT R&D, the role of the health ministry should be enforced. Despite the small portion of MOHW in the whole government investment on HT R&D, MOHW should lead HT R&D mainly through an RBH project. As the final goal of HT R&D is to overcome diseases and to improve health, which is a major mission of MOHW, HT R&D needs to be coordinated according to the priorities of disease control targets and health promotion of the government, which is decided by MOHW. For this purpose, a governmental coordination body, or an HT R&D committee, is required for the increase of efficiency and effectiveness of HT R&D. It should be responsible for determining priorities of HT R&D target items, in collaboration with relevant experts and related government officials from MOHW, Ministry of Education Science and Technology, and Ministry of Knowledge Economy.
Figures and Tables
References
1. Korea Health Industry Development Institute (KHIDI). Hospital Medi-Cluster Strategy for Medical Industry. 2006.
2. Lee JH. Promotion of translational research and medi-cluster formation in Korea: the research hospital program, policy brief 2008-1. 2008. Science and Technology Policy Institute.
3. Kwon KS, Kim KY, Kim NS. Public health research methodology. 2004. Key Chook Press.
4. Ministry of Health and Welfare in Republic of Korea. 5 years' strategic action plan for health technology R&D. 2009. Ministry of Health and Welfare in Republic of Korea.
5. The Perryman Group. A comprehensive assessment of the impact of the University of Texas M. D. 2009. Anderson Cancer Center on business activity in the Houston area and Texas.
6. Ministry of Health and Welfare in Republic of Korea. Mission report on the successful cases of leading oversea research based hospitals. 2010. Ministry of Health and Welfare in Republic of Korea.
7. Task Force Team of MOHW on Research-based Hospital in Republic of Korea. Strategy for the improvement of capability in hospital's research. 2010.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download