Abstract
This study evaluated cancer risk for adult residents near Nuclear Power Plants (NPPs) in Korea through a valid prospective cohort study during 1992-2010. The study cohort was composed of 11,367 adults living within a five km radius from the NPPs for the exposed and 24,809 adults for the non-exposed or reference cohort set at two different levels of proximity; 5-30 km radius and more than 30 km radius away from NPPs. In 303,542.5 person-years of follow-up, a total of 2,298 cancer cases of all sites, or 1,377 radio-inducible cancers diagnosed during 1992-2008 were ascertained. Multiple adjusted hazard ratios and 95% confidence intervals were estimated using multivariate Cox proportional hazard model. There were no epidemiological evidence for increased risk of cancer due to radiation from NPPs. Radiological study results or surveillance data of radiation doses around NPPs could be well documented for risk estimation of radio-inducible cancers, instead of epidemiological study results of the long-time required. Continuous surveillance of quantitative measures of dose levels around NPPs and radiation exposures to the residents is warranted.
There has been public concern over cancer risk for people living near nuclear facilities. Increased cancer risk near nuclear installations was first reported in 1983 (1). Higher incidence of childhood leukemia has been reported in the environs of Sellafield and Dounreay fuel reprocessing plants in the United Kingdom (2, 3). However, the UK Government Committee on the Medical Aspects of Radiation in the Environment (COMARE) concluded that the causes remained unknown (3-5), but were unlikely to involve radiation exposures, to explain the increased incidences because radiation emission from these facilities were estimated to be far below the dose received from natural background dose. Other ensuing studies (6-8) found no difference in cancer risk or no association with nuclear facilities, even in young people.
Numerous epidemiological studies from various countries that investigated cancer risk in populations nearby nuclear facilities have been reported. To date, however, there is no conclusive or consistent epidemiological evidence to support increased cancer risk in those populations. In contrast to the reports (9-22) that showed an increased risk for leukemia, certain specific cancers, and/or all cancers, other studies (23-30) showed negative results. The most frequent study types or designs undertaken were ecological or cross-sectional and a few case-control. Few cohort studies were undertaken.
In Korea, the first nuclear power plant (NPP) has been in operation since April of 1978 in Kori (KR) in Yangsan county. In 1983 two more NPPs have been in operation in KR and Wolsung (WS) county. And during 1985-1989 six more NPPs (two in KR, two in Youngkwang [YG] county, two in Uljin [UJ] county) started to operate. During 1995-1999, seven additional NPPs (three in WS, two in YG, two in UJ), two additional NPPs in 2002 in YG, and two additional NPPs in 2004 and 2006 in UJ have been in operation. Thus, a total of nine NPPs as of 1992, and 20 NPPs as of 2010 in four sites (KR, WS, YG, and UJ) have been in operation by the Korea Hydro & Nuclear Power Co., Ltd (31). And since the startup of nuclear power plants, the radiation doses and exposure in nuclear power facilities, nearby areas, and nearby residents have been strictly monitored by the Korean Institute of Nuclear Safety (KINS) under the supervision of the Ministry of Knowledge Economy.
An episodic case of anencephaly abortus experienced in a YG county resident was reported in a daily newspaper in July, 1989 (32), leading to a possible health hazard risk near NPPs. Press reports that described a possible health risk in populations living near NPPs became an important public health issue in Korea. The government tried to give the public a clear grasp of the matter by dealing with radiation doses monitored by the KINS. However, it was incomprehensible to the public. Eventually, a valid epidemiological study was needed to account for possible cancer risk among residents near NPPs (33).
The Korea Radiation Effect & Epidemiology Cohort (KREEC) study was thus initiated in 1992 to evaluate cancer risk in radiation workers at NPPs and residents near NPPs in Korea (34-37). The resident cohort study (KREEC-R) was completed in February of 2011. The main purpose of the KREEC-R was to evaluate cancer risk due to radiation emitted from NPPs in Korean adult residents near NPPs compared to non-exposed or reference residents.
The exposed cohort of the KREEC-R was adult residents living within five km radius from the NPPs (exposed study area) in four counties, KR, WS, YG, and UJ. The five km bands accorded with the Korean statutory limits of the 'around area' of power plants according to the 'Act on Support to the around Area of Power Plants' (the law number; 10499) enacted in June 1989. The non-exposed, or reference, cohort was set at two different levels of proximity: inter-mediate proximate group were residents living within 5-30 km radius from the NPPs (control-1 study area); far-distance group lived more than 30 km radius away (control-2 study area), i.e., Yangpyong (YP) county, Haman (HM) county (HM), and Choongju (CJ) city (CJ) areas. KREEC-R study was conducted by a collaborative study group of eight research teams from eight institutions, seven universities and the National Cancer Center.
Despite the KINS has monitored radiation doses, an independent radiological investigation in the residential areas near NPPs, the exposed study areas, was conducted by our study group during 1992-1994. The three-months cumulative dose of the γ ray in air was monitored for three years, 1992-1994, by Thermo-luminescence Dosimeter (TLD) at 7-8 spots in each exposed study areas. The mean values of the dose monitored were 22.9-42.3 mR that is, not higher than the background level. And for radiation exposure in residents, the average radiation dose of whole-body exposure was estimated to be 0.25-15.6 µSv/year, which is far below (0.1%-6.2%) the limited dose, 0.25 mSv/year. Our radiological study results showed nearly no difference from the KINS monitored results (38).
The eligible population for the cohort study consisted of all residents aged ≥ 20 yr in the seven study areas. In the exposed study areas, the estimated number of the eligible population was about 33,150 in 1990. To screen potential participants, questionnaires and health examination surveys, i.e. baseline examination, were undertaken yearly during 1992-1994, and 1998-2005 in the seven study areas. A field medical office and clinic in each survey area was opened during a defined period that was about a week to allow publicity for the baseline survey. All visitors to the field clinic underwent free medical check-up and were interviewed via questionnaires after informed signed consent.
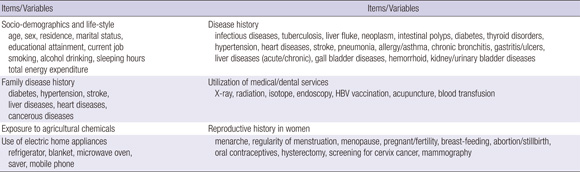
Trained interviewers conducted face-to-face personal interviews, which collected data on baseline characteristics including socio-demographic, life-style, past medical history and family medical history, history of certain environmental exposures, and reproductive history for women, etc (Annex Table 1).
A medical check-up program conducted by the medical team was provided to all visitors. The medical check-up program included simple medical examinations, chest radiography, clinical laboratory tests using blood or urine sample (Annex Table 2). For suspected high risk cancer subjects, additional medical instrumental examinations and some specific assays for cancers were performed (Annex Table 2). Blood and urine samples were obtained and assayed according to standardized procedures. Quality-control procedures were achieved in agreement with the Korean Association of Laboratory Quality Control.
Of a total of 61,651 baseline examinations (18,691, 23,359, and 19,601 in exposed, control-1 and -2 study areas, respectively), 6,329 examinations (3,278 in exposed and 3,051 in non-exposed study areas), were duplicated or repeated examinations within a one to seven year time period. Of the 6,329 duplicated baseline examinations, 477 examinees took the baseline examination repeatedly within a year interval. Using their repeated measurements of the baseline characteristics, reliability of the baseline survey data was evaluated and reported (39). Most of the possible covariates showed good to moderate agreement.
This dynamic prospective cohort was established from 1992-2005. The primary enrollment for the cohort included the following criteria: 1) age ≥ 20 yr, 2) no past or current cancer case, 3) correct residential address, 4) signed informed consent, 5) complete/correct personal identification number (PIN), 6) no considerable missing information. One year after the primary enrollment, recruitment of each subject as cohort member was finalized according to a supplementary criterion, i.e. to have the first one year of follow-up observation conducted.
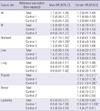
Finally, during 1992-2005 a total of 36,176 baseline examinees, 14,994 men and 21,182 women, were enrolled in the KREEC-R study population or cohort, 11,367 exposed and 24,809 non-exposed (Table 1).
The follow-up study in terms of cancer case ascertainment was conducted during 1992-2010. Annually both passive and active follow-up surveillance were integrated. The Resident database of the Ministry of Public Administration & Security, the Beneficiary database of the National Health Insurance, and the mortality data of the Statistics Korea were used to identify living subjects in Korea. The Korea Central Cancer Registry, death certificate database and medical records from hospitals were used to detect cancer cases defined as C00~D09 according to the International Classification of Diseases-10 (ICD-10).
For completeness of cancer case ascertainment, however, the incident date of the last cancer case ascertained was limited to the end of 2008. Therefore, December 31, 2008 is the cut-off date of follow-up.
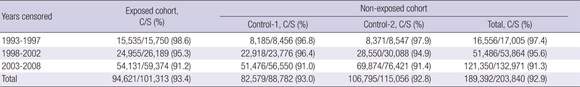
The completion rate of the follow-up surveillance in terms of censoring during 1993-1997 was about 98%, and during 2003-2008, about 91%. Completion of censoring during the entire period, 1993-2008, was 93.1% (Annex Table 3).
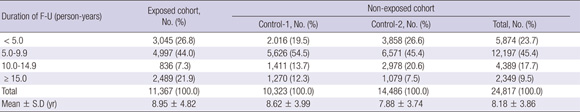
The mean duration of follow-up observation was 9.0 ± 4.82 yr, and 8.2 ± 3.86 yr in the exposed and non-exposed groups, respectively (Annex Table 4). Distribution of follow-up duration is shown in Annex Table 4.
The total follow-up duration was 303,542.5 person-years (PY), 129,607.4 in men and 173,935.1 in women. In the exposed cohort, the follow-up duration was 101,183.3 PY; 43,458.1 for men and 57,698.2 for women. For the non-exposed group, the total follow-up period was 202,359.2 PY; 86,122.3 for men and 116,236.9 for women; 88,707.3 PY in Control-1 study area (Control-1 cohort) and 113,651.9 PY in Control-2 study area (Control-2 cohort). Table 2 shows the follow-up duration by 'age at observation'.
Suspicious cancer cases were screened in the annual follow-up observation, and then cancer cases were identified. Two methods of screening were implemented. Active follow-ups such as personal or telephone contacts or questionnaires were mailed in each study area, and ascertained from the National Medical Claims Data of the National Health Insurance. C00-D09 code cases from the National Medical Claims Data were selected as suspicious cancer cases. Cases with codes other than C00-D09 (other codes) were excluded from the screening, since a trial sample survey on the probability of having cancer among those cases yielded nil. The trial sample survey was conducted with a total of 1,459 claims with other codes in 1,036 members during 2003-2007. The study sample was selected by a stratified weighted sampling, where the weighted variables, i.e. large/university hospital, admission care, and anti-cancer drug prescribed, were drawn by a model function fitted for cancer detection probability among C00-D09 cases in 2003.
Three sources of data for cancer case ascertainment, cancer registry data, death certificate data, and medical record abstracts, were utilized. Cancer codes and dates were identified from cancer registry or death certificate data. Active medical records check-ups of suspicious cancer case were performed. Trained abstractors or medical recorders in the hospital drew up the structured medical record abstract form. Medical doctors reviewed the structured abstracts to assess cancer case, including diagnosis of date, diagnostic measures and interpretation of the results, cancer stage, pathological findings, treatment, etc.
The date of cancer diagnosis was determined as the date of cancer incidence. Cancer code and date were linked to the cancer registry or death certificate data, whichever diagnosis date was earlier.
The radiogenic or radio-inducible cancers (40) were defined as cancers of the stomach (C16), liver (C22), lung (C33-34), bone (C40-41), breast (C50), thyroid (C73), and multiple myeloma (C90), and leukemia (C91-95).
Completeness of cancer case ascertainment for this study was evaluated using cancer cases between 2004 and 2007, ascertained by two-source capture-recapture method and log-linear models in three-source model (41). Completeness calculated in all three sources combined was 96.9% in exposed and 97.1% in non-exposed with no statistically significant difference (P = 0.84).
To evaluate the follow-up duration in terms of power estimation, sample size calculation was performed using PASS 2002 based on Cox regression and Poisson regression model with the assumption of relative risk = 1.3 or 1.5, exposed: non-exposed = 1:2, and power = 0.8 or 0.9. Results of the sample size calculation have been published elsewhere (42). Based on a Cox regression model, relative risk = 1.3 and power = 0.9, a total of 560 outcome events, or 188,389 PY, in the non-exposed cohort was required.
To compare incidence levels of cancer between groups, e.g. exposed, non-exposed, Control-1, and Control-2, the age standardized rates (ASR) in each group as the summary rates were calculated using World Standard Population (43).
Potential confounders or covariates were estimated by correlation analyses with study areas, cancer incidence, and inter-variables. Other than the variable of age at observation, a total of nine variables in males and six variables in females were selected as covariates and were included in the final analysis model. Age adjustment was performed by using Time-updated Cox model.
In the main analyses, hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using multivariate Cox proportional hazard model. All analyses were adjusted for age as a time-varying variable and smoking (men only), alcohol drinking (men only), liver disease history (men only), current job, education attainment, total energy expenditure per day, family history of cancer, body mass index (BMI), and medical services of x-ray or radiation as time-fixed variables. All analyses were performed using SAS 9.2.
During 303,542.5 person-years of follow-up, a total of 2,298 cancer cases of all sites (All), or 1,377 radio-inducible cancers (RI) diagnosed during 1992-2008 was ascertained. In males, 1,334 All, or 832 RI cases were ascertained during 129,607.4 PY of follow-up. And in females, 964 All, or 545 RI cases were ascertained during 173,935.1 PY. In the exposed cohort, 705 All (393 men, 312 women) or 429 RI cases (250 men, 279 women) were ascertained. 1,593 All (941 men, 652 women) or 943 RI cases (57 8 men, 365 women) in the non-exposed cohort were ascertained. In Control-1, 721 All (421 men, 300 women) or 420 RI cases (248 men, 172 women), and in Control-2, 872 All (520 men, 352 women) or 523 RI cases (330 men, 193 women) were ascertained. Table 3 illustrates the distribution of age and year of cancer diagnosis and cancer sites of RI.
Table 4 illustrates the crude rate (CR) and ASR per 100,000 PY of All and RI in the exposed and non-exposed. In males, the ASR of All was 564.7 in the exposed cohort and 544.1 in the non-exposed. In females, it was 306.5 and 281.0, respectively. For RI cancers in males, the ASR in the exposed cohort was 363.0 and 347.4 in the non-exposed cohort, and in females it was 190.5 and 161.9, respectively. ASRs in the non-exposed cohort for Control-1 and Control-2 are also shown in Table 4. ASR's in Korean general population reported by the Korea Central Cancer Registry (44, 45) in 2005-2007 were lower than in the KREEC-R study population. This difference can be explained by the fact that the KREEC-R study population is limited to subjects of age 20 yr or older.
For All or RI (total), there was no increased or decreased risk in the exposed cohort compared to the non-exposed cohort, in either men or women (Table 5). However, HRs by some specific cancer sites increased or decreased cancer risks for a few cancer sites, i.e. stomach and liver in men, and lung and thyroid in women in the exposed cohort. Stomach cancer risk in men was significantly increased compared to the non-exposed (total) and Control-1 (HR = 1.6 and 1.9, respectively). An increased risk for liver cancer in men was also observed compared to Control-1 only. In contrast in women, a decreased risk for lung cancer was observed compared to Control-1 only. While, living near NPPs was positively associated with thyroid cancer risk (HR = 1.9 and 2.5 compared to the non-exposed (total) and Control-2 cohort, respectively).
HRs for the association between the degree of proximity of place of residence to NPPs and radio-inducible cancer risk are shown in Table 6. Except thyroid cancer in women, no association or trend in risk with proximity to NPPs was observed. Thyroid cancer incidence in women in the exposed and Control-1 was as high as 2.5 and 1.8 times, respectively, than in Control-2 cohort. And the trend in risk was statistically significant (P for trend = 0.03).
For the possible influence of radiation from NPPs on risk of radio-inducible cancers, specifically stomach and thyroid cancers, additional analyses were performed using the exposed cohort data. Table 7 provides HRs for the association between duration of living with NPPs and RI cancer risk. No association or trend in risk for duration of living with NPPs was observed (P for trend > 0.05).
Our radiological study conducted in 1992-1994 in residential areas near NPPs showed levels of radioactivity in air and radiation exposure in residents is far below the limited doses, just as monitored by the KINS, and suggested that it is least likely for the nearby residents to have increased cancer risk due to radiation emitted from the NPPs. However, the account for a possible cancer risk by dealing with radiation doses monitored or estimated is hardly acceptable to the public, since method of radiation dose estimates and the limited dose are not well understood by the public. Furthermore, estimated radiation doses in residents near NPPs usually contain un-quantified uncertainties which result in uncertainties with estimated cancer risks (46). Hence, it should not be prudent to dismiss radiation exposures as a possible cause because official or estimated doses are too low. To the public, on the other, epidemiological study results or evidence are easily understandable. And the valid epidemiological study results could render radiation doses inferable.
The most definitive type of observational epidemiologic study is usually the prospective cohort study design (47). However, very few prospective cohort studies have investigated cancer risks, of all sites or radio-inducible cancers, in relation to living near NPPs. This type of study is a typical prospective cohort study, where study subjects (i.e. cohort members) are initially free of the disease under study (i.e. cancer) when examined at baseline, and are classified according to whether they are exposed or not exposed to the possible risk factor (i.e. living near NPPs). The cohort is then followed over time to ascertain cancer incidence.
This study was designed and was conducted to examine increased cancer risk due to radiation exposures for adult residents near NPPs in Korea. Several aspects of the quality of the study such as completion rate of follow-up surveillance, reliability of the covariates, completeness of cancer case ascertainment, and sample size requirements are strengths of the study (39, 41, 42). The study results indicated incidence rates of all sites of cancer or all radio-inducible cancers for the exposed cohort group (adult residents near NPPs) were not significantly different from the sub-cohorts (control-1 or inter-mediate proximate cohort, control-2 or far-distance cohort, and non-exposed or the subcohorts combined) in both men and women. Overall, no increased risk for all sites of cancer or all the radio-inducible cancers was found in this study.
In Table 5, meanwhile, cancer risks of certain specific sites for the residents near NPPs increased or decreased, which was restricted to one gender or a specific comparison cohort. However, these results provide no indication that adults living near NPPs have an increased risk of any radio-inducible cancers, since the results are self-contradictory.
Table 6 indicates any radio-inducible cancer has no association or trend in risk with proximity to NPPs, except thyroid cancer risk in women. A possible explanation for the statistically significant trend in thyroid cancer risk in women with proximity to NPPs would be the influence of un-quantified or un-measured radiation emitted from NPPs. However, Table 7 suggested, though the sample size was not large to provide solid evidence, radiation emitted from NPPs was not associated with increased risks of radio-inducible cancers in nearby residents, even thyroid cancer in women. The probable factors other than radiation from NPPs remain unknown in this study, since this study was not designed to investigate factors other than radiation. Other factors, if needed, are to be investigated through another or supplementary studies.
Consistent with other studies (3-8, 23-30), our study shows no evidence of an increased risk of radio-inducible cancers in adult residents within a five km radius of the NPPs. Our findings support radiological study results could be well documented for cancer risk estimation due to radiation. Continuous surveillance of quantitative measures of dose levels around NPPs and radiation exposures to the residents is warranted, along with radio-ecological studies.
In conclusion, this is a valid prospective cohort study from 1992-2010 evaluated cancer risk among adult residents who reside near NPPs in Korea. There is no epidemiological or causal evidence for increased risk of cancer due to radiation from NPPs. Radiological study results or surveillance data of radiation doses around NPPs could be well documented for cancer risk estimation, instead of epidemiological studies that require a long follow-up period.
Yoon-Ok Ahn, MD (Seoul National University, Seoul, Korea), Zhong-Min Li, MD (Seoul National University, Seoul, Korea), Myung-Chul Lee, MD (Gachon University, Incheon, Korea), Keun-Young Yoo, MD (Seoul National University, Seoul, Korea), Byung-Joo Park, MD (Seoul National University, Seoul, Korea), Joon-Ki Chung, MD (Seoul National University, Seoul, Korea), Jin-Q Kim, MD (Kunkuk University, Seoul, Korea), Chae-Eun Lee, MD (Inje University, Busan, Korea), Duk-Hee Lee, MD (Kyungpook National University, Daegu, Korea), Jin-Soo Choi, MD (Chonnam National University, Gwangju, Korea), Hyun-Sool Lim, MD (Dongkuk University, Pohang, Korea), Jin-Kyung Oh, PhD (National Cancer Center, Goyang, Korea), Bo-Youl Choi, MD (Hanyang University, Seoul, Korea), Soung-Hoon Chang, MD (Kunkuk University, Chungju, Korea).
Figures and Tables
Table 1
Distribution of exposed and non-exposed cohort members by sex, age at enrollment, and year of enrollment

Table 2
Distribution of follow-up period (person-year) of exposed and non-exposed cohort by sex and age group

Table 4
Crude (CR) and age standardized (ASR) cancer incidence rate per 100,000 person-years by sub-cohort group

References
1. Black D. Independent Advisory Group. Investigation of the possible increased incidence of cancer in West Cumbria. 1983. London: Her Majesty's Stationery Office (HMSO).
2. Darby SC, Doll R. Fallout, radiation doses near Dounreay, and childhood leukemia. BMJ. 1987. 294:603–607.
3. Committee on Medical Aspects of Radiation in the Environment (COMARE). First report. The implications of the new data on the release from Sellafield in the 1950s for the conclusion of the Report on the Investigation of the Possible Increased Incidence of Cancer in west Cumbria. 1986. London: HMSO.
4. Committee on Medical Aspects of Radiation in the Environment (COMARE). Second report. Investigation of the possible increased incidence of leukemia in young people near the Doureay Nuclear Establishment, Caithness, Scotland. 1988. London: HMSO.
5. COMARE. Fourth report. The incidence of cancer and leukemias in young people in the vicinity of the Sellafield site, West Cumbria: further studies and an update of the situation since the publication of the report of the Black Advisory Group in 1984. 1996. London: Department of Health.
6. Heasman MA, Kemp JW, Urquhart JD, Black R. Childhood leukemia in Northern Scotland. Lancet. 1986. 1:266.
7. Gardner MJ. Review of reported Increases of childhood cancer rates in the vicinity of nuclear installations in the UK. J R Stat Soc Ser A Stat Soc. 1989. 152:307–325.
8. Gardner MJ, Hall AJ, Downes S, Terrel JD. Results of case-control study of leukemia and lymphoma among young people near Sellafield nuclear plant in West Cumbria. BMJ. 1990. 300:423–429.
9. Cook-Mozaffari OJ, Ashwood FL, Vincent T, Forman D, Alderson M. Cancer incidence and mortality in the vicinity of nuclear installations in England and Wales 1959-1980. Office of Population Censuses and Surveys (OPCS report). 1987. London: HMSO.
10. Forman D, Cook-Mozaffari PJ, Darby S, Davey G, Stratton I, Doll R, Pike M. Cancer near nuclear installations (commentary to the OPCS report). Nature. 1987. 329:499–505.
11. Cook-Mozaffari PJ, Darby SC, Doll R, Forman D, Hermon C, Pike MC, Vincent T. Geographical variation in mortality from leukemia and other cancers in England and Wales in relation to proximity to nucear installations, 1969-1978. Br J Cancer. 1989. 59:476–485.
12. Clapp RW, Cobb S, Chan , Walker B Jr. Leukaemia near Massachusetts nuclear power plant. Lancet. 1987. 2:1324–1325.
13. Bithell JF, Dutton SJ, Draper GJ, Neary NM. Distribution of childhood leukaemias and non-Hodgkin's lymphomas near nuclear installations in England and Wales. BMJ. 1994. 309:501–505.
14. Black RJ, Sharp L, Harkness EF, McKinney PA. Leukaemia and non-Hodgkin's lymphoma: incidence in children and young adults resident in the Dounreay area of Caithness, Scotland in 1968-91. J Epidemiol Community Health. 1994. 48:232–236.
15. Pobel D, Viel JF. Case-control study of leukemia among young people near La Hague nuclear reprocessing plant: the environmental hypothesis revisited. BMJ. 1997. 314:101–106.
16. Sharp L, McKinney PA, Black RJ. Incidence of childhood brain and other non-haematopoietic neoplasms near nuclear sites in Scotland, 1975-94. Occup Environ Med. 1999. 56:308–314.
17. Gulis G, Fitz O. Cancer incidence around the Nuclear Poweer Plant Jaslovske Bohunice. Cent Eur J Public Health. 1998. 6:183–187.
18. Draper GJ, Stiller CA, Cartwright RA, Craft AW, Vincent TJ. Cancer in Cumbria and in the vicinity of the Sellafield nuclear installation, 1963-90. BMJ. 1993. 306:89–94.
19. Silva-Mato A, Viana D, Fernandez-SanMartin MI, Cobos J, Viano M. Cancer risk around the nuclear power plants of Trillo and Zorita (Spain). Occup Environ Med. 2003. 60:521–527.
20. Mangano JJ, Sherman J, Chang C, Dave A, Feinberg E, Frimmer M. Elevated childhood cancer incidence proximate to U.S. nuclear power plants. Arch Environ Health. 2003. 58:74–82.
21. Kaatsch P, Spix C, Schulze-Rath R, Schmiedel S, Blettner M. Leukemia in young children living in the vicinity of German nuclear power plants. Int J Cancer. 2008. 122:721–726.
22. Spix C, Schmiedel S, Kaatsch P, Schulze-Rath R, Blettner M. Case-control study on childhood cancer in the vicinity of nuclear power plants in Germany 1980-2003. Eur J Cancer. 2008. 44:275–284.
23. Darby SC, Doll R. Fallout, radiation doses near Dounreay, and childhood leukemia. BMJ. 1987. 294:603–607.
24. Kinlen L. Evidence for an infectious cause of childhood leukaemia: comparison of a Scottish new town with nuclear reprocessing sites in Britain. Lancet. 1988. 2:1323–1327.
25. Enstrom JE. Cancer mortality patterns around the San Onofre nuclear power plant 1960-1978. Am J Public Health. 1983. 73:83–92.
26. Poole C, Rothman KJ, Dreyer NA. Leukaemia near Pilgrim nuclear power plant, Massachusetts. Lancet. 1988. 2:1308.
27. Crump KS, Ng TH, Cuddihy RG. Cancer incidence pattern in Denver metropolitan area in relation to the Rocky Flats plant. Am J Epidemiol. 1987. 126:127–135.
28. Jablon S, Hrubec Z, Boice JD Jr, Stone BJ. Cancer in populations living near nuclear facilities. 1990. Bethesda, Md: Public Health Service, Dept of Health and Human Services;90–874. NIH Publication.
29. Clarke EA, McLaughin J, Anderson TW. Childhood leukemia around Canadian nuclear facilities - Phase I, Final Report. 1989. Ottawa, Ontario: Atomic Energy Control Board.
30. Viel JF, Richardson ST. Childhood leukemia around La Hague nuclear facilities. Health Physics. 1989. 56:875–884.
31. Korea Hydro & Nuclear Power Co., LTD. accessed on 7 February 2011. Available at http://www.khnp.co.kr.
32. Dong-A Ilbo. July 31, August 2, 1989.
33. Repubic of Korea, Parliament Office. Stenographic records of the 1989 Parliamentary (Committee on Science and Economy) inspection of the Ministry of Science and Technology. 1989. September 21 and October 4.
34. Ahn YO, Lee MC, Yoo KY, Chung JK, Park BJ, Li ZM, Kim JQ, Lee CE, Lee DH, Choi JS, et al. Epidemiological investigation on cancer risk among radiation workers in nuclear power plants and residents nearby nuclear power plants in Korea (the final report). 2011. Ministry of Education, Science and Technology (MEST No. 2010-0000986).
35. Jeong MS, Jin YW, Yang KH, Ahn YO, Cha CY. Radiation exposure and cancer incidence in a cohort of nuclear power industry workers in the Republic of Korea, 1992-2005. Radiat Environ Biophys. 2010. 49:47–55.
36. Cardis E, Vrijheid M, Blettner M, Gilbert E, Hakama M, Hill C, Howe G, Kaldor J, Muirhead CR, Ahn YO, et al. Risk of cancer after low doses of ionizing radiation: retrospective cohort study in 15 countries. BMJ. 2005. 331:77–82.
37. Bae JM, Yang YJ, Li CM, Ahn YO. Low cholesterol is associated with mortality from cardiovascular diseases: a dynamic cohort study in Korean adults. J Korean Med Sci. 2011. 27:58–63.
38. Korean Institute of Nuclear Safety. Reports of Evaluation and Investigation for the radiation exposure in nuclear facilities and surrounding areas. 1990, 1992-1996.
39. Bae SH, Park BY, Li CM, Ahn YO. Reliability of covariates in baseline survey of a cohort study: epidemiological investigation on cancer risk among residents who reside near the nuclear power plants in Korea. J Prev Med Public Health. 2010. 43:159–165.
40. Stewart BW, Kleihues P, editors. World Cancer Report. 2003. Lyon: IARC Press;51–53.
41. Song MK, Cho IS, Li CM, Ahn YO. Completeness of cancer case ascertainment in Korea radiation effect and epidemiology cohort study. J Korean Med Sci. 2012. 27:489–494.
42. Cho IS, Song MK, Choi YH, Li CM, Ahn YO. Power estimation and follow-up period evaluation in Korea radiation effect and epidemiology cohort study. J Prev Med Public Health. 2010. 43:543–548.
43. Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, Boyle P. Cancer Incidence in Five Continents IX. IARC Scientific Publications. 2008. 160:111–112.
44. Jung KW, Won YJ, Park SH, Kong HJ, Sung JH, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2005. J Korean Med Sci. 2009. 24:995–1003.
45. Jung KW, Park SH, Kong HJ, Won YJ, Boo YK, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010. 25:1113–1121.
46. Fairlie I. Childhood cancer near nuclear power stations. Environ Health. 2009. 8:43.
47. Kelsey JL, Thompson WD, Evans AS. Methods in observational epidemiology. 1986. New York: Oxford University Press;8–9.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download