Abstract
The triad of rash, arthritis, and uveitis seems to be characteristic for early-onset childhood sarcoidosis. We describe an interesting case of early-onset childhood sarcoidosis coexisting enchondromatosis, which clinically masquerade as Langerhans cell histiocytosis. A 33 months old girl presented with skin rash, subcutaneous nodules with polyarthritis, and revealed the involvement of lymph nodes as well as spleen during work-up. She also presented with multiple osteolytic lesions which pathologically proven enchondromatosis. Oral prednisone was prescribed at 2 mg/kg/day for 2 months until when subcutaneous nodules and joint swellings almost disappeared, and then slowly tapered over a period of 5 months. We report an unusual case of early-onset childhood sarcoidosis presented with osteolytic bone lesions which were irrelevant to sarcoidosis.
Sarcoidosis is a multisystem granulomatous disease of unknown cause (1). There are two distinct forms of childhood sarcoidosis. In older children, clinical manifestations are similar to the adult, such as frequent lymphadenopathy and pulmonary involvement. The early-onset form exhibit unique clinical triad of arthritis, rash, and uveitis, which typically presented in the first year of life (2-5).
The early-onset childhood sarcoidosis may be overlooked due to its similarity to juvenile rheumatoid arthritis (JRA) (6). Both entities may be associated with arthritis and skin rashes. Arthritis of sarcoidosis is characterized by painless boggy effusions of synovium without limitation of motion (2, 7). Although painful and destructive polyarthritis with functional impairment indistinguishable from that associated with JRA has been described in sarcoidosis (3), bone involvement is quite rare in sarcoidosis and JRA patients. In contrast, osteolytic bone lesions without synovial involvements usually develop in children with Langerhans cell histiocytosis (LCH), enchondromatosis, leukemia/lymphoma, and metastatic neuroblastoma.
Here we report a child presented with polyarthritis, subcutaneous nodules, skin rashes, and osteolytic bone lesions. Although the clinical presentation was masqueraded as LCH, the biopsy findings were compatible with sarcoidosis for skin rash, subcutaneous nodule, synovium and coexisting enchondromatosis for bone lesion.
A 33-months old girl with limping gait and multiple joint swelling was referred to our hospital in November, 2008. She was born in Korea and has stayed at the Philippines for several months with her parents. She presented knee joint swelling since 2 months ago, when visited clinic in the Philippines and received simple radiographic evaluation which revealed no definite abnormalities. However, her joint swelling has been persisted and accompanied by intermittent skin rashes, and she referred to our hospital with the radiologic evidence of osteolytic lesions on the fibula which taken in another hospital of Korea.
Physical examination revealed diffuse swellings on the wrist, ankle, and knee joints with tenderness. The palpable multiple nodular lesions on the both wrists, and the maculopapular skin eruptions on the trunk and extremity were noted. The multiple bean-sized lymph nodes were palpable only on the cervical area, not on the axillar and inguinal area. The liver tip was palpated just below the right costal margin and spleen was not. Cardiac, respiratory, ocular, and neurological examinations were unremarkable. Routine blood tests revealed hemoglobin 9.3 gm/dL, white blood cell 8,600/µL, platelet 309,000/µL, ESR 62 mm/hr, CRP 3.5 mg/dL, AST 23 U/L, ALT 15 U/L, total bilirubin 0.1 mg/dL, LDH 226 U/L, alkaline phosphatase 104 U/L.
In the plain radiographies, the osteolytic lesions surrounded by a thin sclerotic rim were noted on the both femoral necks, both proximal fibula, right distal fibula, and left distal tibia (Fig. 1). MRI demonstrated diffuse synovial enhancement in left knee and right ankle joints. In addition, there were signal intensity alterations on the distal femur, proximal/distal fibula, proximal/distal tibia, as well as 2nd metatarsal bone, 2nd to 4th proximal phalanges, and tarsal bones (Fig. 2). The whole body bone scan showed no definite abnormal focal uptakes except mildly increased soft tissue uptake on the bilateral suprapatellar bursa area (Fig. 3). We also performed PET-CT scan which demonstrated hypermetabolic soft tissue uptakes around both knee and ankle joints. In addition, hypermetabolic lymph nodes were also noted in bilateral cervical, parapharyngeal, and mid-abdominal area as well as palatine tonsils (Fig. 4).
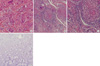
Multiple biopsies were done on the skin rash, subcutaneous nodule, synovium, and bone. The biopsy findings revealed the non-caseating, sarcoidal granulomas in the skin rash and subcutaneous nodule, as well as synovium of knee joint. However, the pathologic finding of right tibia was compatible with enchondroma (Fig. 5).
We diagnosed this patient with early-onset childhood sarcoidosis accompanied by incidentally noted enchondromatosis. We also performed direct gene sequencing analysis for nucleotide binding oligomerization domain 2 (NOD2) which revealed no genetic mutation and there is no family history of early-onest sarcoidosis. Oral prednisone was initiated at 2 mg/kg/day for 2 months until when subcutaneous nodules and joint swellings almost disappeared, and then slowly tapered over a period of 5 months. Hypermetabolic lesions in cervical area, nasopharynx, both palatine tonsils were also almost disappeared in the PET-CT scan during induction treatment of oral prednisone.
Sarcoidosis is a systemic granulomatous disease of unknown etiology. Because sarcoidosis affects most organs, the clinical presentation can vary greatly. The disease frequently involves the lungs, lymph nodes, eyes, skin, liver, and spleen (8-11). There are two distinct forms of childhood sarcoidosis. In older children, multisystem manifestations similar to the adult usually develop, such as frequent lymphadenopathy and pulmonary involvement as well as generalized signs and symptoms. In contrast, early-onset childhood sarcoidosis, i.e., with onset in the first 5 yr of life differs from sarcoidosis in older children and adults. Patients with early-onset childhood sarcoidosis exhibit unique clinical triad of arthritis, rash, and uveitis, which typically presented in the first year of life (5, 8).
In our patient, clinical diagnosis at presentation was very difficult because osteolytic lesions on the long bones were coexisted with the evidence of arthritis. Several disease categories including early-onset sarcoidosis, Langerhans cell histiocytosis, JRA, lymphoma, should be considered, although the synovial involvement in LCH and osteolytic bony lesions in JRA and sarcoidosis are extremely rare (12, 13). Particularly, systemic LCH coexisting with polyarthritis was highly suspected before performing biopsy in consideration of the age of patient, multiple osteolytic lesions, and skin rashes. We could confirm the diagnosis of early-onset childhood sarcoidosis with enchondromatosis by the histologic findings.
Uveitis, which occurs in about in more than half the children with early-onset childhood sarcoidosis (3), was not developed in our patient. However, hypermetabolic lesions on PET-CT scan were noted in the reticuloendothelial systems including lymph nodes and spleen, which almost disappeared after induction therapy. Although we did not perform the biopsy on the lymph nodes or spleen, we could suspect that the reticuloendothelial systems were also affected in our patient, since PET-CT scan can be used to assess the extent and metabolic response to systemic treatment of inflammatory diseases, such as sarcoidosis (14-16).
Bone involvement is quite rare and its frequency varies from 1% to 13% in large published series of sarcoidosis patients. Furthermore, bone involvement is rarely reported in early-onset childhood sarcoidosis because it usually occurred in patients with chronic course or known multisystem sarcoidosis (13). In our patient, initial osteolytic bone lesions were proved as enchondromatosis by the pathology.
In conclusion, we report an unusual case of early-onset childhood sarcoidosis presented with osteolytic bone lesions which were irrelevant to sarcoidosis. Therefore, despite the rare occurrence of early-onset childhood sarcoidosis, it should be considered in the differential diagnosis when a child presents with arthritis, skin rashes, and even bony lesion. However, pathologic confirmation would be also needed to confirm diagnosis because the incidental bony lesions could coexist like our case. PET-CT scan may be used to identify the extent of sarcoidosis and the treatment response.
Figures and Tables
Fig. 1
Radiological images of the patient. (A) Multiple, small and elongated osteolytic lesions with thin sclerotic rims are noted in the both femoral necks (arrows). Both knee AP (B) and lateral views (C, D) show small, eccentric osteolytic lesions involving right proximal fibula and left proximal fibula (white arrows). Large amount of suprapatellar effusions are also noted in the both sides of knee (black lined white arrows). Both ankle AP (E) and right lateral views (F) show well-defined osteolytic lesions with thin sclerotic rim involving right distal fibula (white arrows) and left distal tibia (black lined white arrows).
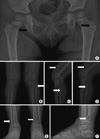
Fig. 2
MR scan images of low extremity. Contrast enhancement T1-weighted axial (A) and sagittal scans (B) with fat-suppression show diffuse synovial enhancement (white arrows) and tenosynovitis (black lined white arrows) around right ankle joint. Abnormal enhancement lesion is also noted in the right 2nd metatarsal shaft (black arrow). Contrast enhancement T1-weighted sagittal scans (C) with fat-suppression show joint effusion with diffuse synovial enhancement (white arrow) in the left tibia MR scans.
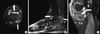
Fig. 3
The whole body bone scan showed no definite abnormal focal bony uptake lesions except mildly increased soft tissue uptake on the bilateral suprapatellar bursa area (arrows).
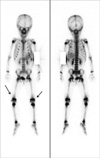
Fig. 4
PET/CT images. (A) Whole body MIP (maximum intensity projection) image. (B) Regional images demonstrating hypermetabolic activity in nasopharynx, bilateral tonsils and cervical lymph nodes. (C) Mid abdominal lymph nodes. (D) Thickened synovial lining of both knee joints.
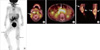
Fig. 5
Histopathological findings of the biopsied tissue. Non-caseating, sarcoidal, chronic granulomatous inflammation is observed in the skin rash (A) and subcutaneous nodule, and synovium of knee joint (B, C). However, the pathological finding of right tibia is compatible with enchondroma (D). (H&E stained, A × 200; B, × 100; C and D, × 200).
References
1. Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, Eklund A, Kitaichi M, Lynch J, Rizzato G, Rose C, Selroos O, Semenzato G, Sharma OP. ATS/ERS/WASOG statement of sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999. 16:149–173.
2. Hetherington S. Sarcoidosis in young children. Am J Dis Child. 1982. 136:13–15.
3. Häfner R, Vogel P. Sarcoidosis of early onset. A challenge for the pediatric rheumatologist. Clin Exp Rheumatol. 1993. 11:685–691.
4. Fink CW, Cimaz R. Early onset sarcoidosis: not a benign disease. J Rheumatol. 1997. 24:174–177.
5. Pattishall EN, Kendig EL Jr. Sarcoidosis in children. Pediatr Pulmonol. 1996. 22:195–203.
6. Mallory SB, Paller AS, Ginsburg BC, McCrossin ID, Abernathy R. Sarcoidosis in children: differentiation from juvenile rheumatoid arthritis. Pediatr Dermatol. 1987. 4:313–319.
7. Rosenberg AM, Yee EH, MacKenzie JW. Arthritis in childhood sarcoidosis. J Rheumatol. 1983. 10:987–990.
8. Hoffmann AL, Milman N, Byg KE. Childhood sarcoidosis in Denmark 1979-1994: incidence, clinical features and laboratory results at presentation in 48 children. Acta Paediatr. 2004. 93:30–36.
9. Baculard A, Blanc N, Boulé M, Fauroux B, Chadelat K, Boccon-Gibod L, Tournier G, Clement A. Pulmonary sarcoidosis in children: a follow-up study. Eur Respir J. 2001. 17:628–635.
10. Fauroux B, Clèment A. Paediatric sarcoidosis. Paediatr Respir Rev. 2005. 6:128–133.
11. Kim DS. Sarcoidosis in Korea: report of the Second Nationwide Survey. Sarcoidosis Vasc Diffuse Lung Dis. 2001. 18:176–180.
12. Aouba A, Larousserie F, Le Guern V, Martin A, Guillevin L. Spumous histiocytic oligoarthritis coexisting with systemic Langerhans' cell histiocytosis: case report and literature review. Joint Bone Spine. 2009. 76:701–704.
13. Wilcox A, Bharadwaj P, Sharma OP. Bone sarcoidosis. Curr Opin Rheumatol. 2000. 12:321–330.
14. Nishiyama Y, Yamamoto Y, Fukunaga K, Takinami H, Iwado Y, Satoh K, Ohkawa M. Comparative evaluation of 18F-FDG PET and 67Ga scintigraphy in patients with sarcoidosis. J Nucl Med. 2006. 47:1571–1576.
15. Teirstein AS, Machac J, Almeida O, Lu P, Padilla ML, Iannuzzi MC. Results of 188 whole-body fluorodeoxyglucose positron emission tomography scans in 137 patients with sarcoidosis. Chest. 2007. 132:1949–1953.
16. Zhuang H, Alavi A. 18-fluorodeoxyglucose positron emission tomographic imaging in the detection and monitoring of infection and inflammation. Semin Nucl Med. 2002. 32:47–59.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download