Abstract
Henoch-Schönlein purpura (HSP) is common in childhood and often self-limiting. There have been limited studies on elderly-onset HSP nephritis (HSPN). A 76-yr-old man was transferred to our hospital with a 1-month history of oliguria, abdominal pain, edema and palpable purpura in the legs. Three months ago, he was admitted to another hospital with jaundice, and consequently diagnosed with early common bile duct cancer. The patient underwent a Whipple's operation. Antibiotics were administrated because of leakage in the suture from the surgery. However, he showed progressive renal failure with edema and purpura in the legs. Laboratory investigations showed serum creatinine 6.4 mg/dL, 24-hr urine protein 8,141 mg/day, myeloperoxidase anti-neutrophil cytoplasmic antibodies (MPO-ANCA) 1:40 and C3 below 64.89 mg/dL. Renal biopsy showed crescentic glomerulonephritis, as well as mesangial and extracapillary Ig A deposition. We started steroid therapy and hemodialysis, but he progressed to end-stage renal failure and he has been under maintenance hemodialysis. We describe elderly onset HSPN with MPO-ANCA can be crescentic glomerulonephritis rapidly progressed to end stage renal failure.
Henoch-Schönlein purpura (HSP) is a common systemic vasculitic disorder that occurs during hildhood and its course is often self-limiting (1, 2). Approximately 40% patients with HSP develop HSP nephritis (HSPN). Multiple studies have reported less frequent but poorer outcomes in adult HSPN (1, 2) and there have been a few data on elderly-onset patients (3). HSPN and Immunoglobulin A (IgA) nephropathy are considered to be related diseases. IgA nephropathy is usually an indolent progressive adult disease, whereas HSPN is mainly seen during childhood. HSPN presents extrarenal manifestations and has associations with hypersensitivity. HSPN more often shows nephritic-nephrotic syndrome and chronic renal failure compared to IgA nephropathy (4).
Although the pathophysiologic mechanisms of HSPN are not completely understood, thecomplement activation, vascular damage and crescent formation in glomerular injury in severe HSPN appear to play an important role. However, the serum complement levels are usually normal (5) and anti-neutrophil cytoplasmic antibodies (ANCA) is negative in most patients with HSPN (2, 3, 6). We experienced a case of elderly-onset crescentic HSPN with nephritic-nephrotic syndrome associated with hypocomplementemia and positive myeloperoxidase ANCA (MPO-ANCA) after a major operation and antibiotic use. Renal failure was not improved and the patient has been under maintained HD.
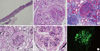
A 76-yr-old man was transferred to our hospital at 7th July 2011 with 1-month history of oliguria, gross hematuria, abdominal pain, pitting edema and skin eruption (palpable purpura) in both legs. He had no previous medical history with normal renal function and urine analysis findings. His family history was also unremarkable. Three months ago, he was admitted to another hospital with jaundice, but then diagnosed with early common bile duct cancer, and he underwent Whipple's operation. Antibiotics were used because of leakage in the suture from the surgery but there were no further surgical problems. The antibiotics administered included ceftriaxone, amikacin, meropenem and piperacillin/tazobactam. However, serum creatinine increased from 1.0 to 6.3 mg/dL. A physical examination revealed multiple nontender purpura mixed with erythema ranging from 2 to 4 mm in diameter over his edematous lower legs and the dorsal side of his feet and toes (Fig. 1). Most of the skin lesions were non-indurated, although palpable or indurated purpura mixed with erythema could be identified in some areas. On admission, laboratory investigations showed a reduced serum hemoglobin level of 10.5 g/dL and an elevated serum C-reactive protein level of 5.93 mg/dL. In addition to an elevated serum creatinine level of 6.4 mg/dL (Fig. 2), an increased blood urea nitrogen level of 45.7 mg/dL, urine RBC many/HPF and 24 hr urine protein showed 8,141 mg/day. Urine electrophoresis suggested nonselective glomerular proteinuria. C3 was low at 64.89 mg/dL, and ANCA was positive at MPO-ANCA 1:40 (by enzyme-linked immunosorbent assay [ELISA] and indirect inmunofluorescence [IF]) and serum fluorescent antinuclear antibody test, cryoglobulin and other serology findings were within the normal range. Ultrasonography showed diffuse parenchymal swelling, homogenous increase cortical echogenecity, ascites and bilateral pleural effusion. Renal biopsy showed crescentic glomerulonephritis on light microscopy (LM), and mesangial and extracapillary Ig A deposition on IF. We examined eight glomeruli, two of which were globally sclerotic. Circumferential fibrocellular and cellular crescents were noted in 5 out of a total of 8 glomeruli. Most glomeruli were diffuse endocapillary proliferation in size and cellularity due to endocapillary proliferation with mild lymphocytic and neutrophilic infiltration. IF microscopy revealed conspicuous staining for Ig A in the mesangium and segmentally subendothelial portion along the glomerular capillary walls (Fig. 3). On the 10th hospital day, the patient complained of abdomen discomfort and experienced pyrexia up to 38.5℃. We started prednisolon 60 mg without using antibiotics, and after that the fever improved. Starting steroid therapy and HD, abdomen discomfort, purpura on legs, pretibial pitting edema and pleural effusion were improved. However, his renal function was not improved and he has been under maintenance HD.
HSP is a typical disease of childhood, and shows a near 20-fold disparity in incidence between adults and children (1.3 vs 22.1 per 100,000 population). Adult-onset HSP may involve more severe HSPN and organ injury, but there is limited data on elderly-onset HSP (3). The classic clinical symptoms include purpuric rash, abdominal pain, arthralgias, and hematuria, but the spectrum of HSP may vary from only minimal petechial rash to severe gastrointestinal, neurologic, pulmonary, and renal disease. Approximately 40% with HSP develop HSPN. HSPN is often triggered by antecedent infection, medication and other miscellaneous factors (8). HSPN and IgA nephropathy are currently considered related diseases, as both are indistinguishable in the renal histologic and immunofluorescent findings, and both have been described in twins. HSPN more often shows extrarenal manifestation, nephritic-nephrotic syndrome and chronic renal failure compared to IgA nephropathy (4).
Pathogenesis of HSPN is not completely understood, but it has pathologically been regarded as a specific immune-mediated entity induced by environmental factors, particularly infections (9). The role of infection, particularly group A beta-hemolytic streptococcal, in the etiology of HSP has interested many investigators, but the role of bacterial antigens remains questionable in a majority of cases. Other causes are hypersensitivity reactions induced by numerous drugs, but no common drug has yet been identified. In a previous case report, prodromal illness and drug to HSP in three members of the same family were penicillin with sore throat and colon resection after diverticulitis with sulfonamide due to wound infection (10).
Pathophysiologic mechanisms of HSPN including complement activation, vascular damage and crescent formation in glomerular injury appear to play an important role. C3 deposits are seen in a vast majority of patients with HSPN. Hypocomplementemia generally has not been seen in HSPN, but excessive consumption and deposition of C3 in the kidneys may have been the cause of reduced C3 serum level in a severe HSPN case (5). HSP is a systemic vasculitic disorder. ANCA with specificity for myeloperoxidase (MPO) was noted in primary small vessel vasculitis (11). Although the prognosis of patients with overlapping HSPN with ANCA remains unclear, clinical correlations suggest that immune complex deposits in the kidney may potentiate the effect of ANCA in producing more severe glomerulonephritis than classic pauci-immune vasculitis or HSPN. As HSPN overlapping syndrome characterized by the synergistic effect of MPO-ANCA and the IgA immune complex may result in a fatal outcome, aggressive immunosuppressive therapy should be initiated as early as possible (12). We examined MPO-ANCA twice using an enzyme immunoassay (ELISA) in our hospital and indirect IF assay in another hospital. It was found that MPO-ANCA was positive and a titer of 1:40 was relatively weak. However, large multicenter studies combining indirect IF and ELISA have demonstrated a specificity approaching 99% for Wegener's granulomatosis, microscopic polyangitis, or their renal-limited variant (13). The ANCA have an important role in pathogenicity of small vessel vasculitis, and intermittent ANCA positivity is an independent risk factor for relapse (11-13). Therefore, MPO-ANCA may have some role in the pathogenesis of our crescentic HSPN case. In a retrospective study, 2 cases with ANCA out of a total of 42 HSP could not identify any clinical correlation between HSP and ANCA (6). Because the presence of crescents is a histologic feature of HSPN that represents a prognostic factor and constitutes the basis for the International Study Group of Kidney Disease in Childhood (ISKDC) pathology classification, the study of crescent pathophysiology may provide useful information as a therapeutic strategy (14).
A cohort study (3) of 250 adults suffering from HSP was retrospectively analyzed for a median follow-up period of 14.8 yr. The risk of progression to renal insufficiency, which ranges from 5% to 15% in children, seems to be much higher in adults, at approximately 30%. They reported to define long-term prognosis and risk factors for severe renal failure in adults with HSPN. Renal function impairment and proteinuria level at presentation and, the degree of interstitial fibrosis, percentage of sclerotic glomeruli, and presence of glomeruli with fibrinoid necrosis on renal biopsy were associated with a poor renal prognosis.
Our elderly patient underwent a kidney biopsy-proven, sudden developed HSPN associated with nephritic-nephrotic syndrome with hypocomplementemia and p-ANCA after a major operation for the treatment of cancer. Renal biopsy showed crescentic glomerulonephritis indicating a poor prognosis. After being initially treated with prednisolone, his purpura and edema on the legs improved, but renal function did not improve. This case implies that the prognosis of elderly-onset crescentic HSP nephritis with positive MPO-ANCA and hypocomplentemia may be a poor outcome. Therefore, closed inspection would be helpful to monitor the progression of elderly-onset HSP, and the clinician should not delay renal biopsy to confirm the diagnosis at the time of clinical suspicion of renal involvement. Earlier diagnosis and prompt treatment of elderly-onset HSPN will prevent the progress of end-stage renal failure. Only few cases with elderly onset HSPN have been reported, especially rare with MPO-ANCA. We describe elderly onset HSPN with MPO-ANCA can be crescentic glomerulonephritis rapidly progressed to end stage renal failure.
Figures and Tables
Fig. 1
Skin rash and generalized edema were showed on lower extremities. Palpable purpuric rash mixed with erythema on both edematous legs (A) and feet (B).
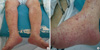
Fig. 2
Changes in serum creatinine concentration in the patient during the hospital course. HD, hemodialysis; op, operation; CT, computerized tomography.

Fig. 3
Microscopic findings of needle biopsy specimen. (A) Eight glomeruli, 2 of which are globally sclerotic, whereas 5 showed segmental sclerosis. (B) Glomerulus shows fibrocellular crescent with focal capsular tear on PAS staining. (C) Masson's trichrome stain shows golmerular sclerosis with fibrin deposition and interstitial fibrosis. (D) The silver staining shows some infiltrated neutrophils. The glomerular capillary basement membrane is not thickened. (E) The glomerulus demonstrates diffuse endocapillary proliferation with circumferential cellular crescent formation formation more than 50% glomeruli (Meadow classification Grade V, Jones silver stain, original magnification × 400). (F) Immunofluorescence demonstrates diffuse deposition of IgA in the mesangium and subendothelial portion of the glomerulus (anti-IgA immunofluorescence, original magnification × 400).
References
1. Blanco R, Martínez-Taboada VM, Rodríguez-Valverde V, García-Fuentes M, González-Gay MA. Henoch-Schönlein purpura in adulthood and childhood: two different expressions of the same syndrome. Arthritis Rheum. 1997. 40:859–864.
2. Pillebout E, Thervet E, Hill G, Alberti C, Vanhille P, Nochy D. Henoch-Schönlein purpura in adults: outcome and prognostic factors. J Am Soc Nephrol. 2002. 13:1271–1278.
3. Diehl MP, Harrington T, Olenginski T. Elderly onset Henoch-Schönlein purpura: a case series and review of the literature. J Am Geriatr Soc. 2008. 56:2157–2159.
4. Davin JC, Berge IJ, Weening JJ. What is the difference between IgA nephropathy and Henoch-Schönlein purpura nephritis. Kidney Int. 2001. 59:823–834.
5. Krause I, Garty BZ, Davidovits M, Cleper R, Tamary H, Rosenmann E, Eisenstein B. Low serum C3, leukopenia, and thrombocytopenia: unusual features of Henoch-Schönlein purpura. Eur J Pediatr. 1999. 158:906–909.
6. Kim NH, Ham YR, Yoon JH, Jung JY, Kim ES, Chung S, Choi DE, Na KR, Lee KW, Shin YT. Henoch-Schönlein nephritis in adults: renal outcomes and prognostic factors. Korean J Nephrol. 2009. 28:570–578.
7. Shrestha S, Sumingan N, Tan J, Alhous H, McWilliam L, Ballardie F. Henoch-Schönlein purpura with nephritis in adults: adverse prognostic indicators in a UK population. QJM. 2006. 99:253–265.
8. Rai A, Nast C, Adler S. Henoch-Schönlein purpura nephritis. J Am Soc Nephrol. 1999. 10:2637–2644.
9. Yang YH, Chuang YH, Wang LC, Huang HY, Gershwin ME, Chiang BL. The immunobiology of Henoch-Schönlein purpura. Autoimmun Rev. 2008. 7:179–184.
10. Lofters WS, Pineo GF, Luke KH, Yaworsky RG. Henoch-Schönlein purpura occurring in three members of a family. Can Med Assoc J. 1973. 109:46–48.
11. Bollée G, Noël LH, Suarez F, Royal V, Gilardin L, de Serre NP, El-Ghoul B, Lesavre P, Alyanakian MA, Fakhouri F. Pauci-immune crescentic glomerulonephritis associated with ANCA of IgA class. Am J Kidney Dis. 2009. 53:1063–1067.
12. Nagasaka T, Miyamoto J, Ishibashi M, Chen KR. MPO-ANCA- and IgA-positive systemic vasculitis: a possibly overlapping syndrome of microscopic polyangiitis and Henoch-Schöenlein purpura. J Cutan Pathol. 2009. 36:871–877.
13. Bartůnková J, Tesar V, Sedivá A. Diagnostic and pathogenetic role of antineutrophil cytoplasmic autoantibodies. Clin Immunol. 2003. 106:73–82.
14. Davin JC. Henoch-Schönlein purpura nephritis: pathophysiology, treatment, and future strategy. Clin J Am Soc Nephrol. 2011. 6:679–689.
15. Hauer HA, Bajema IM, van Houwelingen HC, Ferrario F, Noël LH, Waldherr R, Jayne DR, Rasmussen N, Bruijn JA, Hagen EC. Renal histology in ANCA-associated vasculitis: differences between diagnostic and serologic subgroups. Kidney Int. 2002. 61:80–89.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download