Abstract
Echinococcal disease can develop anywhere in the human body. The liver represents its most frequent location. Hepatic hydatid cysts may rupture into the biliary tract, thorax, peritoneum, viscera, digestive tract or skin. We report a rare case with rupture of the right hepatic duct into a hydatid cyst in a woman with known hydatid disease and choledocholithiasis. The increased intra-luminal pressure in the biliary tree caused the rupture into the adjacent hydatid cyst. The creation of the fistula between the right hepatic duct and the hydatid cyst decompressed the biliary tree, decreased the bilirubin levels and offered a temporary resolution of the obstructive jaundice. Rupture of a hydatid cyst into the biliary tree usually leads to biliary colic, cholangitis and jaundice. However, in case of obstructive jaundice due to choledocholithiasis, it is possible that the cyst may rupture by other way around while offering the patient a temporary relief from his symptoms.
Hydatid disease, although rare, is still endemic in many countries, representing an important public health problem. It may occur in any organ or tissue. Reviews show that the most frequently involved organs are the liver (55%) and lungs (40%). Rarely, hydatid disease may be found in the spleen (1.8%), kidneys (1.4%) bones (0.1%) or other sites (1.7%). Multiple simultaneous locations are observed in 25% of cases (1, 2).
A common complication of hepatic hydatid disease is the rupture of the cyst caused by the increased pressure within it (2). Cysts may rupture into the biliary tree, the peritoneal or pleural cavity, the pericardium, the gastrointestinal tract, or even into blood vessels. Echinococcal cysts may coexist with cholelithiasis or scarcely with choledocholithiasis (3).
The aim of this study was to present an unusual case of hydatid cyst coexisting with choledocholithiasis and obstructive jaundice, in which the increased intraluminal pressure in the right hepatic duct led to the decompression of the biliary tree in to the cystic cavity. Diagnostic methods and various treatment modalities were also discussed.
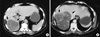
A 60-yr-old female, coming from a rural area, was referred in March 2009 to the Emergency Department complaining for right upper quadrant pain and jaundice. She was diagnosed with echinococcal cyst of the liver and cholelithiasis four years ago. The laboratory examinations on admission were: white blood cell count (WBC): 10,000/µL without eosinophilia; lactate dehydrogenase (LDH) 1,209 µ/L; serum glutamic oxaloacetic transaminase (SGOT) 256 µ/L; serum glutamic pyruvic transaminase (SGPT) 401 µ/L; total bilirubin 5.5 mg/dL with direct bilirubin 3.6 mg/dL. Abdominal ultrasound revealed multiple echinococcal cystic lesions in the right lobe of the liver. Additionally, the gallbladder was distended, containing multiple small stones and mud, while the intrahepatic biliary tree and the common bile duct were dilated. The subsequent computed tomography (CT) showed a huge multisegmented hydatid cyst of the right lobe occupying the segments V, VI, and VII, The intra- and extra-hepatic biliary trees were dilated (Fig. 1). She was treated conservatively, her transaminasemia and hyperbilirubinemia subsided and the patient was discharged seven days later.
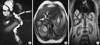
Ten days later, she was readmitted with jaundice. Her biochemical and hematological tests revealed severe transaminasemia and hyperbilirubinemia (SGOT 358 µ/L; SGPT 491 µ/L; LDH 754 µ/L; bilirubin 12.7 mg/dL with direct bilirubin 8.3 mg/dL). Surprisingly, an acute fall in her bilirubin levels was noticed two days later (LDH 400 µ/L; SGOT 175 µ/L; SGPT 335 µ/L; gamma-glutamyltransferase (γ-GT) 786 µ/L; alkaline phosphatase (ALP) 461 µ/L; bilirubin 5.4 mg/dL with direct bilirubin 3,2 mg/dL). Magnetic resonance cholangiopancreatography (MRCP) was performed showing a communication between the echinococcal cyst and the right hepatic duct, while the common hepatic duct and the common bile duct were full with material of unknown origin (Fig. 2).
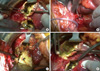
A surgical intervention was decided upon. An open cholecystectomy, with exploration of the common bile duct was performed. Multiple gallstones were removed from the common bile duct. Additionally, partial cystectomy was performed. Surprisingly, pigmented gallstones with daughter cysts were found and removed from the echinococcal cystic cavity (Fig. 3). The intra-operative cholangiogram revealed communication between the echinococcal cyst and the right hepatic duct. The right hepatic duct was ligated. The cystic cavity and the subhepatic space were drained, and a T-tube was placed into the common bile duct. The patient recovered well from the operation and her postoperative course was uneventful. After discharge, albenazole was administered for 3 month cycles, with 14 day intervals. Her follow-up included ultrasonography and CT scan 3 months and 12 months after the operation. Two years after the operation the patient was free of disease and symptoms.
Hydatid cysts grow at a variable rate. They may stabilize, or become calcified, while others may collapse or even completely resolve (4). Becoming symptomatic may be due to pressure exertion of the cyst on the liver parenchyma, or rupture into surrounding tissues (3). When referring to biliary tract, the rupture is common in the right and left hepatic duct and rarely may occur into the hepatic duct junction, common bile duct or cystic duct (5). Scarcely, the hydatid cyst could perforate into the gallbladder (5).
Hydatid cyst rupture has been classified into three types: i) contained when only the endocyst ruptures and the cyst contents are confined within the pericyst; ii) communicating when the cyst contents escape via biliary radicles and iii) direct when both the endocyst and the pericyst tear, allowing cyst contents to spill into the pleural or peritoneal spaces (6, 7). In the present case a communicating rupture of the echinococcal cyst occurred causing a fistula between the echinococcal cyst and the right hepatic duct. As a result, the biliary tree was decompressed, serum bilirubin levels were decreased and the patient improved. The communicating type of rupture is the most frequent complication, representing approximately 50% of cases on admission (6). The mechanism of intrabiliary rupture seems to be that of entrapment of small bile duct radicles in the pericyst, which due to increased intracystic pressure undergo atrophy resulting in rupture (8). Following cyst enlargement, communication with larger ducts is established (8). Most hydatid cysts of the liver eventually leak into small bile ducts or perforate into larger ones. In large surgical series, some sort of communication was found in 40%-90% of cysts (9). In our case, the progressive growth of the cyst led its wall to be adjacent to the right hepatic duct, while choledocholithiasis and subsequent obstruction increased the pressure inside the biliary tree. This combination resulted in the rupture of the right hepatic duct into the hydatid cyst to decompress the biliary tree and as a result a biliocystic fistula was established.
Intrabiliary rupture can occur with two different clinical settings which are followed by certain symptoms. These are occult communication (10%-37%) and frank intrabiliary rupture (3%-17%) (10). The occult rupture is usually silent and may be accompanied by suppuration or can evolve towards a frank rupture (9). In the frank rupture daughter vesicles and fragmented membranes escape into the biliary tree causing obstruction, cholangitis or septicemia (11). The present case was probably an occult communication, which evolved towards a frank rupture.
The diagnosis of intrabiliary ruptured hydatid cysts has been assisted by imaging tests. Echogenic material, without posterior acoustic shadowing in the extrahepatic ducts, is a finding implying the presence of intracystic material (12). Abdominal CT scan may reveal the dilated common bile duct with low attenuation intraluminal material, suggesting the presence of hydatid sand and cysts (13). Recently, magnetic resonance imaging (MRI-MRCP) has proven to be a useful noninvasive diagnostic modality in cases of intrabiliary rupture, whereas CT scan and ultrasound results are inconclusive (13).
Endoscopy is a modality serving both diagnostic and therapeutic aims. During preoperative endoscopic retrograde cholangiopancreatography (ERCP), daughter cysts may be seen in the duodenum, impacted in the ampulla of Vater or obstructing any part of the biliary tree (14, 15). Moreover, postoperative ERCP may resolve obstruction or cholangitis due to residual material in biliary ducts, while providing management of postoperative external biliary fistulae (15). Additionally, endoscopic sphincterotomy has proved to be an alternative treatment for patients with biliary hydatid disease (12).
In case of cystobiliary communication a surgical intervention is mandatory. Various types of procedures have been proposed such as: partial cystectomy with primary closure, partial cystectomy with drainage, cystotomy with drainage, hepatic resection (atypic, segmentary or lobar) and omentoplasty (9). Suturing of the cystobiliary fistula, and if feasible common bile duct exploration using intraoperative cholangiography are also required (11). If the biliary tract is cleaned of all cystic content, a T-tube drain is usually sufficient.
In conclusion, rupture of a hepatic hydatid cyst into the biliary tree is the most common complication of hydatid disease. Usually, it leads to biliary colic, cholangitis and jaundice. However, it is possible that the rupture is being done conversely, relieving the patient from the obstructive symptoms. Currently, ERCP is a method of both diagnosis and treatment. Further surgical treatment may be required if an obvious communication between the biliary tree and the hydatid cyst is displayed.
Figures and Tables
Fig. 1
CT scan images (A, B) showing a huge multi-locular cyst (arrows) adjacent the A B right hepatic bile duct.
References
1. Dziri C, Haouet K, Fingerhut A, Zaouche A. Management of cystic echinococcosis complications and dissemination: where is the evidence? World J Surg. 2009. 33:1266–1273.
2. Prousalidis J, Tzardinoglou E, Sgouradis L, Katsohis C, Aletras H. Uncommon sites of hydatid disease. World J Surg. 1998. 22:17–22.
3. Lewall DB, McCorkell SJ. Rupture of echinococcal cysts: diagnosis, classification, and clinical implications. AJR Am J Roentgenol. 1986. 146:391–394.
4. WHO Informal Working Group on Echinococcosis. Guidelines for treatment of cystic and alveolar echinococcosis in humans. Bull World Health Organ. 1996. 74:231–242.
5. Manouras A, Genetzakis M, Antonakis T, Lagoudianakis P, Pattas M, Papadima A, Giannopoulos P, Memenakos E. Endoscopic management of a relapsing hepatic hydatid cyst with intrabiliary rupture: a case report and review of the literature. Can J Gastroenterol. 2007. 21:249–253.
6. Marti-Bonmati L, Menor F, Ballesta A. Hydatid cyst of the liver: rupture into the biliary tree. AJR Am J Roentgenol. 1988. 150:1051–1053.
7. Papavramidis TS, Pliakos I, Triantafillopoulou K, Michalopoulos N, Polyzonis M, Sapalidis K, Kesisiglou I, Papavramidis ST. Endoscopic removal of biliary tree echinococcal cysts. Ann Gastroenterol. 2009. 22:116–118.
8. Yalin R, Aktan AO, Yeğen C, Döşlüoğlu HH. Significance of intracystic pressure in abdominal hydatid disease. Br J Surg. 1992. 79:1182–1183.
9. Prousalidis J, Kosmidis C, Kapoutzis K, Fachantidis E, Harlaftis N, Aletras H. Intrabiliary rupture of hydatid cysts of the liver. Am J Surg. 2009. 197:193–198.
10. Erzurumlu K, Dervisoglu A, Polat C, Senyurek G, Yetim I, Hokelek M. Intrabiliary rupture: an algorithm in the treatment of controversial complication of hepatic hydatidosis. World J Gastroenterol. 2005. 11:2472–2476.
11. Spârchez Z, Osian G, Onica A, Bărbânta C, Tantău M, Pascu O. Ruptured hydatid cyst of the liver with biliary obstruction: presentation of a case and review of the literature. Rom J Gastroenterol. 2004. 13:245–250.
12. Mendez Montero JV, Arrazola Garcia J, Lopez Lafuente J, Antela Lopez A, Mendez Fernandez R, Saia Ayala A. Fat-fluid level in hepatic hydatid cyst: a new sign of rupture into the biliary tree? AJR Am J Roentgenol. 1996. 167:91–94.
13. Ozaslan E, Bayraktar Y. Endoscopic therapy in the management of hepatobiliary hydatid disease. J Clin Gastroenterol. 2002. 35:160–174.
14. Zargar SA, Khuroo MS, Khan BA, Dar MY, Alai MS, Koul P. Intrabiliary rupture of hepatic hydatid cyst: sonographic and cholangiographic appearances. Gastrointest Radiol. 1992. 17:41–45.
15. Simşek H, Ozaslan E, Sayek I, Savaş C, Abbasoğlu O, Soylu AR, Balaban Y, Tatar G. Diagnostic and therapeutic ERCP in hepatic hydatid disease. Gastrointest Endosc. 2003. 58:384–389.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download