Abstract
Acute otitis media (AOM) and otitis media with effusion (OME) are common infections in children, and their diagnosis and treatment have significant impacts on the health of children and the costs of providing national medical care. In 2009, the Korean Otologic Society organized a committee composed of experts in the field of otolaryngology, pediatrics, and family medicine to develop Korean clinical practice guidelines (CPG) for otitis media in children with the goal of meeting regional medical and social needs in Korea. For this purpose, the committee adapted existing guidelines. A comprehensive literature review was carried out primarily from 2004 to 2009 using medical search engines including data from Korea. A draft was written after a national questionnaire survey and several public audits, and it was editorially supervised by senior advisors before publication of the final report. These evidence-based guidelines for the management of otitis media in children provide recommendations to primary practitioners for the diagnosis and treatment of children younger than 15 yr old with uncomplicated AOM and OME. The guidelines include recommendations regarding diagnosis, treatment options, prevention and parent education, medical records, referral, and complementary/alternative medicine for treating pediatric otitis media.
Acute otitis media (AOM) and otitis media with effusion (OME) are highly prevalent in the pediatric population and represent major disease burdens worldwide. Following the Korea National Health Survey performed in 2009, otitis media (OM) was ranked as the seventh most frequent disease responsible for hospital visits among patients younger than 18 yr old (1).
AOM is a very common disease in children younger than 3 yr old. Two of three children experience AOM once, and about one in three children could have more than three episodes of AOM. The prevalence of AOM in Korea has not been reported, although previous studies have revealed AOM incidence rates of 62% and 83% in children under 1 and 3 yr old, respectively (2). The incidence rate differs according to age; the incidence is low in the neonatal period but rises markedly after 6 months old, peaking at around 2 yr old, with another slightly lower peak from 4 to 7 yr old. Despite its considerable economic impact, there have been no prospective studies regarding the incidence of AOM in Korea. According to the 2008 statistics of the Health Insurance Review and Assessment Service, the yearly cost of AOM was 140 billion Won, which is 9.3% that of acute respiratory tract infections.
Following a nationwide survey of Korean children under 15 yr old, the prevalence rates of AOM and OME were reported to be 0.08% and 1.22%, respectively (3). The prevalence of OME in Korean kindergarten children has been reported as 10.8-16.4% (4-8). Two of these studies reported that 90% of affected children did not display subjective symptoms (5, 7). OME also has major medical, social, and economic consequences. The disease course typically takes months and may occur repeatedly during childhood, when functional maturation of the Eustachian tube is incomplete. Furthermore, it can cause hearing loss, which may delay language/speech acquisition and cognitive development in the affected child. It can also cause permanent changes in the tympanic membrane, such as cholesteatoma, which can be prevented through a relatively simple surgical intervention. OME also has a marked adverse effect on the quality of life of the affected children and their families (9, 10).
Considering the high prevalence and societal significance of AOM and OME in children, evidence-based guidelines for OM in the pediatric population have been developed in many countries and continue to be updated to reflect new evidence and changing social circumstances (11-15). As medical decisions should be based on the balance between risk and benefit based on existing evidence, national guidelines should reflect evidence from the particular region, and careful modification may be necessary considering the availability of medical facilities and social costs applied in each particular country.
The development of Korean clinical practice guidelines for pediatric OM is necessary and important for several reasons. First, the microbiology and antibiotic resistance of AOM in Korea differ from cases reported in other countries (16, 17). Second, the need for nationally based guidelines became apparent with the publication of the 2004 clinical practice guidelines in the USA (11, 13). Third, a 2010 Korean survey of otolaryngologists concerning the treatment of childhood OM revealed 90% agreement among respondents on the need for Korea-based clinical practice guidelines for pediatric OM.
Thus, the Korean Society of Otology launched a committee in 2009 tasked with the development of Korean clinical practice guidelines for pediatric AOM and OME. The committee developed a set of guidelines for the diagnosis and treatment of OM in children, grounded in evidence-based studies, with the intent of implementation in actual clinical practice.
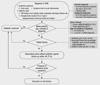
This paper reports the core recommendations of the Korean clinical practice guidelines for pediatric AOM and OME with brief commentary. Algorithms for the diagnosis and treatment of pediatric AOM and OME are shown in Fig. 1 & 2. The full version of the guidelines, in Korean, and full references, are available at the website of the Korean Medical Guideline Information Center (KMGIC) (http://www.guideline.or.kr/guideline/guide/contents.php?number=45&F_sid=974).
The Korean Society of Otology launched a committee to develop clinical practice guidelines for pediatric OM in May 2009. The multidisciplinary committee consisted of seven members from the Korean Society of Otology and two members each recommended by the Korean Society of Pediatrics and the Korean Society of Family Medicine. The development team adapted the 2004 AOM guidelines of the American Academy of Pediatrics (AAP), 2004 OME guidelines of the American Academy of Otolaryngology-Head and Neck Surgery (AAO), and the 2008 OME surgical guidelines of the National Institute for Health and Clinical Excellence (NICE) as optimal models.
The guideline development subcommittee conducted a nationwide survey among otolaryngologists in August 2009 to collect public opinion and investigate the current trends in clinical practice for pediatric OM in Korea as part of the guideline development project. During the development period, the process and preliminary recommendations were presented several times at scientific meetings and public hearings.
For 2 months starting in August 2009, three researchers individually searched resources from 2004 to 2009 using the keywords "acute otitis media," "pathogen," "otitis media with effusion," "ventilation tube," and "antibiotics" using PubMed, Cochrane database, EBSCO, and KoreaMed search engines. Additionally, systematic reviews and references of meta-analyses were also collected. The above-mentioned existing guidelines and 2009 Japanese guidelines for pediatric AOM were reviewed and analyzed (14). The literature search included systematic reviews, existing guidelines, randomized controlled trials, and other types of original papers on clinical issues, with additional material identified manually by subcommittee members. The search covered materials published in English and Korean. The collected evidence was reviewed and assessed using a grading system from Level I (well-designed randomized, controlled trials or meta-analyses) to Level IV (expert opinion, animal studies, in vitro research). The evidence level was downgraded when the study seemed to be biased or the evidence quality was not sufficiently strong to enable generalization of the findings.
The recommendations in the guidelines are based on the best available published data identified by the committee members. Where qualified evidence was lacking, an expert consensus based on clinical experience was used. Each recommendation statement was graded using a modified system based on that used in the 2004 American Academy of Pediatrics guidelines for AOM, which consisted of four levels considering the quality of supporting evidence and clinical significance (Table 1) (12). According to this system, variability in clinical practice is expected to be less with a strong recommendation than that with a recommendation. Options provide the best chances for practice variability.
The final draft guidelines underwent an external review by five otology experts based in both university and private clinics and two methodologists from the KMGIC. The final version of the guidelines was published on October 2010 and released to the public on the KMGIC webpage (http://www.guideline.or.kr). An update of the guidelines was tentatively schedule for 5 yr from publication, or sooner if compelling new evidence warranted revision.
The practice guidelines are for children under 15 yr old, including those under 6 months of age without other underlying diseases or any complications caused by AOM. Those in whom AOM complications were expected (i.e., Down's syndrome, craniofacial anomalies, including cleft palate, and those with immunodeficiencies, cochlear implant patients, patients with AOM that recurred within 30 days, AOM occurrence in a diseased state, or recurrent OM) were excluded; individual management is mandated for such patients.
The clinical practice guidelines are intended to provide evidence-based recommendations to support clinical decisions regarding diagnosis, treatment, and prevention of OME in otherwise healthy Korean children under 15 yr old. Application of these guidelines may be inappropriate for children simultaneously suffering from other co-existing disease(s) or at risk of complication associated with OM. An individualized approach to managing every problem in a comprehensive manner would be preferred in such cases.
Intended users of these guidelines include all physicians in Korea who care for children with AOM and OME, including otolaryngology, pediatrics, and family medicine specialists.
The guidelines are not intended as the sole source for managing children with AOM and OME, but have been designed to assist physicians by providing current evidence regarding OM in children. Clinical findings and environmental factors of each child should be taken into consideration first, and clinical decisions made based on the individual experience of the physician may be more appropriate in certain situations. These guidelines do not provide any considerations for administrative matters or legal issues.
Diagnosis of AOM is made based upon subjective symptoms and objective signs. Subjective symptoms refer to 1) acute onset and 2) middle ear or systemic symptoms due to acute inflammation. Objective signs include 1) tympanic membrane findings, including bulging, bullae, hyperemia, perforation with otorrhea, middle ear effusion (MEE), etc., and 2) tympanometry showing type B or C or identification of MEE via tympanocentesis. "Definite diagnosis" requires both of the subjective symptoms and one or more objective signs; "suspicious diagnosis" is defined as fulfilling all of the subjective symptoms but none of the objective signs (Recommendation grade: A).
The definition of "acute" in the diagnosis of AOM involves abrupt onset of inflammatory symptoms, which are clinically evident within 48 hr and no longer than 3 weeks from onset.
In Japan, Harabuchi et al. (18) defined AOM as acute onset infection of the middle ear with otalgia, fever, and otorrhea, where "acute" is defined as symptoms identified by the child or a parent/caregiver, with medical examination within 48 hr. The diagnosis of AOM is based on an abrupt onset of otalgia and fever with fluid in the middle ear (tympanic membrane bulging, decreased or absent tympanic membrane mobility, fluid level behind the tympanic membrane, or acute otorrhea) and an erythematous membrane.
Local middle ear symptoms due to acute inflammation include otalgia and otorrhea, and systemic symptoms include crying, irritability, fever, and other physical symptoms due to the affected ear (Table 2). Younger age and tympanic membrane findings are highly correlated with severity, and there may be no improvement in the tympanic membrane even though systemic symptoms may have disappeared (19, 20). In these guidelines, "severe" AOM is defined as severe otalgia or irritability lasting longer than 24 hr or when fever exceeds 38.5℃ (Table 3). In a previous study performed in Korea, the tympanic membrane temperature of children suffering from unilateral acute suppurative otitis media was reported as 37.1℃-38.5℃ (mean 37.92℃), and in most cases, ear temperature did not exceed 38℃ (21). Based on these results, "severe" AOM is defined in the guidelines as fever over 38.5℃, but the development team agreed that additional research is needed to clarify this issue.
The age of the patient should also be considered as an important factor to determine treatment options. In children younger than 2 yr of age, Eustachian tube dysfunction and middle ear abnormalities, measured by tympanometry, during upper respiratory tract infections have been reported to be more severe compared with those in older children (22). Among Korean children with type B tympanogram at diagnosis, those older than 4 yr old showed significantly better prognosis than those under 4 yr old (23) (Fig. 1).
The "observation policy" as initial management of AOM refers to watchful waiting for natural improvement for 48 to 72 hr without use of antibacterial agents (Recommendation grade: A).
This policy does not imply neglecting the patient without any treatment. Rather, acute symptoms such as otalgia and fever should be managed. The observation policy should include a follow-up visit after 2-3 days and a decision concerning subsequent treatment options; if symptoms continue, antibiotic therapy should be considered, and a follow-up appointment within 2-3 days should be made. Rosenfeld and Kay (24) also reported "observation" as a treatment option. However, in cases of 1) age under 6 months, 2) definite diagnosis of AOM in a patient under 24 months old, 3) severe AOM, 4) accompanying disease, such as tonsillitis, requiring administration of antibiotics, 5) inability to follow up after 48-72 hr, and 6) recent antibiotic use, the "observation policy" is inappropriate for initial treatment (Table 3). This option without antibacterial treatment should be limited to cases such as 1) non-severe illness, 2) mild cases in patients older than 6 months old or suspected diagnosis for those 6-24 months old, and 3) considering other patient factors (no recent history of taking antibiotics, no other accompanying disease, capability of clinical follow-up after 2-3 days) (25). During conservative therapy without antibiotics for 2-3 days, antiinflammatory agents such as acetaminophen (10-15 mg/kg per dose every 4-6 hr, maximum of five times daily, no more than 75 mg/kg daily) or ibuprofen (5-10 mg/kg per dose every 6-8 hr, maximum of 40 mg/kg/day) are prescribed for mild otalgia and fever. Gastrointestinal distress induced by analgesics can be reduced if they are taken simultaneously with food. In cases managed with conservative therapy, a follow-up visit or telephone contact with the clinician within 48-72 hr is necessary; if this is not possible, antibiotics can be prescribed in advance (26). Tympanocentesis can be performed to relieve severe otalgia when the patient is cooperative, and Gram staining, microbial culture, and antibacterial agent sensitivity studies in these cases can be useful in selecting antibiotics for use in the event of treatment failure (27).
AOM guidelines published in other countries suggest that watchful waiting with symptomatic treatment only constitutes an appropriate treatment option for children with non-severe AOM (11, 14, 15). This observation policy could significantly reduce the carriage of multidrug-resistant organisms and the costs of AOM care. However, parent education is essential for this observation approach to AOM treatment to be successful because many parents expect antibiotics when their child is diagnosed with AOM. Clinicians should also make a correct diagnosis and explain the policy of AOM observation to parents.
Both American and Japanese guidelines emphasize the conservative management of otalgia, with consideration of antibiotic administration by age and symptom severity, and recommended observation with conservative management for older children and mild cases (11, 14, 28). In 2006, Spiro et al. (29) conducted a randomized controlled trial of 283 children with AOM, in which patients were randomized into either the WASP group ("wait-and-see prescription" for antibiotics, in which parents were asked not to fill the prescription unless the child either showed no improvement or worsened in 48 hr) or the SP group (standard prescription, antibiotics administered initially). Substantially more parents in the WASP group did not fill the antibiotic prescription (62% vs 13%; P < 0.001). There were no statistically significant differences between the groups in the frequencies of subsequent fever, otalgia, or unscheduled visits for medical care. The WASP approach substantially reduced unnecessary use of antibiotics in children with AOM and may be an alternative to routine use of antimicrobials for treatment of such cases (27, 29). In a randomized controlled trial, there were no statistically significant differences in otalgia or fever between groups with and without antibiotic prescription (30).
We recommend high-dose amoxicillin (80-90 mg/kg/day) as the first-line antibiotic (Recommendation grade: A). However, if the patient is older than 24 months with no recent antibiotic administration and has not been to a childcare facility, the standard dose of amoxicillin (40-50 mg/kg/day) is recommended as the first-line antibiotic. For severe AOM, high-dose amoxicillin/clavulanate (14:1) (80-90/6.4 mg/kg/day) is recommended as the first-line antibiotic, considering the possibility of β-lactamaseproducing Haemophilus influenzae, Moraxella catarrhalis, and penicillin-resistant pneumococci as the causative organisms (11) (Recommendation grade: A).
As the first-line antibiotic therapy, oral high-dose amoxicillin (80-90 mg/kg/day) and oral amoxicillin/clavulanate (14:1) 80-90/6.4 mg/kg/day for severe cases are recommended in the 2004 AAP guidelines and other large-scale, randomized controlled studies (11, 14, 31). However, the amoxicillin/clavulanate 14:1 formula is not available in Korea, and therefore amoxicillin can be added to available amoxicillin/clavulanate to boost the amoxicillin dose to 80-90 mg/kg. For example, in cases where amoxicillin/clavulanate 7:1 formula is used, amoxicillin/clavulanate 40-50/6.4 mg/kg/day dosage plus amoxicillin 40 mg/kg/day can be administered. In cases with amoxicillin/clavulanate 4:1 formula, amoxicillin/clavulanate 23/5.75 mg/kg/day plus amoxicillin 57 mg/kg/day should be prescribed. When prescribing amoxicillin/clavulanate, the clavulanate dosage should not exceed 10 mg/kg/day because the frequency of diarrhea may increase at higher doses (32, 33).
Indications for exceptional administration of antibiotics initially without an observation period were mentioned above (Table 3). Alternative drugs are recommended for children with a history of type I hypersensitivity reaction to penicillins (e.g., urticaria, anaphylaxis), including macrolides. For non-type I hypersensitivity reactions, cephalosporins are recommended as the first-line antibiotics (11). The standard antibiotic administration period is 10 days for moderate and severe cases, but 5-7 days is possible in mild cases with close observation of the antibiotic response and disease progression recommended after 2-3 days of antibiotic use (34, 35).
These recommendations for the first-line antibiotics are supported by previous studies on antimicrobial susceptibility of pathogenic bacteria in Korea. In a study in pediatric patients with community-acquired respiratory infections due to Streptococcus pneumoniae in Korea, standard-dose aminopenicillin treatment did not result in different outcomes between susceptible and resistant groups (17). However, in a recent study using a pharmacodynamics model, high-dose oral amoxicillin was predicted to be effective against 82.6% of S. pneumoniae isolates from healthy Korean children, whereas the standard-dose regimen was effective in only 30.4% of cases (36).
In randomized controlled trials performed in the USA comparing the effects of amoxicillin/clavulanate and azithromycin on nasopharyngeal bacterial carriage in children with AOM, significant decreases in carriage of S. pneumoniae and H. influenzae were observed in both groups, but the effects were more pronounced in the amoxicillin/clavulanate treatment group (37, 38). In 2008, a study investigating macrolide use in Korea showed that the clinical use of macrolides led to the emergence of bacterial resistance, and especially azithromycin, which has a longer half-life, was closely related to the emergence of resistance to other macrolide antibiotics. As S. pneumoniae isolated from Korean children also showed rapid increases in macrolide resistance, careful consideration is required when choosing antibiotics for treating AOM in children (16). In a 2011 study, susceptibility to erythromycin was reported as 4.3% among S. pneumoniae isolates from healthy Korean children (36).
The routine use of other medications, such as antihistamines and decongestants, for treating AOM in children was shown to lack clinical benefits in a recent Cochrane review (39).
We recommend oral amoxicillin/clavulanate (14:1) 80-90/6.4 mg/kg/day as second-line antibiotic therapy. In cases in which second-line antibiotic therapy fails, parenteral administration of 50 mg/kg/day of ceftriaxone for 3 days is recommended as third-line antibiotic therapy (Recommendation grade: A). However, when the results of the antibacterial agent sensitivity test are available, the most appropriate type of antibiotic can be chosen at any time after obtaining the result (Recommendation grade: B).
Antibiotic treatment failure refers to a lack of improvement in symptoms, including otalgia, fever, and otorrhea, after 48-72 hr of antibiotic administration. Persistent symptoms, including rhinorrhea, cough, or OME, should not be regarded as treatment failure. Infection with organisms resistant to the first-line antibiotics, viral infection alone, or simultaneous infection can be considered as causes of treatment failure. OME occurs after AOM in 50% of patients regardless of treatment period and initial antibiotic selection. OME was reported to persist in 60%-70% of patients after 2 weeks of AOM, 40% after 1 month, and 10%-25% after 3 months. It should not be considered as a complication of AOM, and only observation of disease progression is needed. In these cases of persistent OME, continuous follow-up is required due to bacteriological persistence and the possibility of recurrence, even if symptomatic improvement occurs (40, 41).
Second-line antibiotics are used if no symptomatic improvement is seen after first-line antibiotic administration for 48-72 hr, and third-line antibiotics after failure of second-line antibiotics to elicit an improvement in the patient's condition (11). In the case of antibiotic treatment failure, tympanocentesis for microbial culture with an antibacterial agent sensitivity test should be performed. These procedures and changes in antibiotic can be performed at any time as necessary, but it is best for this to be done before commencement of antibiotic treatment because of the increased possibility of negative bacterial culture results after antibiotic administration. Methods to acquire samples for bacterial culture include tympanocentesis, sampling otorrhea from the external auditory canal with a perforated tympanic membrane, and collecting nasopharyngeal mucus by the transnasal approach (14). If tolerable, tympanocentesis can relieve otalgia in severe cases, and the procedure can help in the selection of antibiotics by a microbial culture with an antibacterial agent sensitivity test after failure of first-line antibiotic treatment (27).
In cases with symptom persistence after macrolide administration in patients with type I hypersensitivity reaction (e.g., urticaria, anaphylaxis) to penicillins, clindamycin can be used as the second-line antibiotic. In cases of non-type I hypersensitivity reactions, cephalosporins such as cefuroxime, cefprozil, cefpodoxime, cefdinir, and cefditoren can be used as first-line antibiotics, and 3 days of parenteral ceftriaxone can be used if symptoms persist. However, as the resistance rate of S. pneumoniae identified in Korea to new macrolides exceeds 70%, bacterial culture is recommended if the child is cooperative, preferably before the first administration of antibiotics (32). Recurrence of disease by the same pathogen was seen in only 28% of cases of recurrent OM after antibiotic treatment.
When examining children diagnosed with AOM, it is recommended that the parent/caregiver be educated concerning the risk factors of AOM to prevent recurrent AOM (Recommendation grade: B).
Groups at high risk for recurrent AOM include children attending child care centers, those under 2 yr old, those with a short breast-feeding history, those who use a milk bottle in the supine position or use pacifiers, those with craniofacial anomalies, and those with passive tobacco smoke exposure. In these instances, education of parents/caregivers to decrease the incidence of recurrent AOM is required. Education of parents/caregivers regarding preventable risk factors, such as preventing upper respiratory tract infections at child care centers and kindergartens, recommending breastfeeding for at least the first 6 months of life, avoiding bottle feeding in the supine position, reducing or eliminating pacifier use in the second 6 months of life, and prohibiting smoking by family members, is important. A study analyzing 257 papers indicated that the risk factors for recurrent AOM were use of pacifiers, attendance at daycare facilities, less breastfeeding history, siblings, craniofacial anomalies, indirect exposure to cigarette smoke, and adenoid vegetations (42).
Injection of pneumococcal conjugate vaccine for prevention of AOM should be decided based on the benefits and risks, the clinician's judgment, and parent's/caregiver's preferences (Recommendation grade: C). However, vaccination is recommended in patients with a high risk of meningitis following AOM, (i.e., children with/expecting a cochlear implant or with congenital inner ear anomalies) (Recommendation grade: A).
Vaccinations such as pneumococcal conjugate vaccine reduce the prevalence of AOM (11, 43). In a retrospective study of the changing patterns of antibiotic resistance rates and clinical features in pneumococcal infections in Korea, pneumococcal conjugate vaccination for prevention of multidrug resistance was proposed (44). In a study performed by Kim et al. (45) in 2004 in a total of 213 children younger than 5 yr old, the pneumococcal nasopharyngeal carriage rate was 34.3%, and 83.8% of S. pneumoniae isolates were resistant to penicillin. Therefore, the authors advocated introduction of pneumococcal conjugate vaccine in Korea (44, 45). After the introduction of the 2008 CLSI (Clinical and Laboratory Standards Institute) standards for antimicrobial susceptibility testing, the ratio of penicillin-susceptible S. pneumoniae changed from 74.7% to 93.2% in the USA. In Korea, penicillin susceptibility of S. pneumoniae has been reported to be 78.3% with the same updated 2008 standards, which suggested a higher resistance rate than in the USA (36).
The United States Centers for Disease Control and Prevention recommends pneumococcal conjugate vaccination of patients with a cochlear implant or congenital inner ear anomalies who are at high risk of cerebrospinal fluid leakage because of the elevated risk of bacterial meningitis (46). Therefore, our guidelines also recommend pneumococcal conjugate vaccine in patients at high risk of meningitis after AOM due to the presence of a cochlear implant and/or congenital inner ear anomalies. However, in cases without these meningitis risk factors, pneumococcal conjugate vaccine injection in AOM should be decided according to the individual benefits and risks considering the clinician's judgment and parent's/caregiver's preferences because insufficient information is available regarding the cost-effectiveness of vaccination for AOM in Korea.
When examining pediatric patients with suspected AOM, the physician should take the patient's history and document the following categories to achieve consistency in future examinations of the individual: 1) onset of AOM symptoms such as otalgia, otorrhea, fever, and irritability; 2) associated symptoms such as rhinorrhea, nasal obstruction, and sore throat; 3) past history of AOM treatment and other diseases; and 4) presence of high-risk factors (Recommendation grade: B).
For the accurate diagnosis of AOM, thorough history taking is required, and the onset of symptoms of otalgia, otorrhea, fever, irritability, and the presence and severity of fever should be determined (19). History taking about past AOM treatment history, antibiotic prescription history, associated diseases, and symptoms is necessary to decide on the use of antibiotics. Even in patients with a past history of AOM, high-risk factors of recurrent AOM (attendance at child care center, age less than 2 yr, lack of breastfeeding history, supine bottle feeding or pacifier use, craniofacial anomalies, and indirect exposure to cigarette smoke) should be identified and documented (11).
Referral to other medical centers, including higher institutes for further evaluation and surgical treatment, is made by the primary clinician during the course of AOM in the following situations: 1) recurrent AOM; 2) tympanic membrane perforation lasting longer than 6 weeks; 3) mastoiditis or subperiosteal abscess; 4) labyrinthitis; 5) facial palsy; 6) spontaneous nystagmus; 7) central nervous system symptoms such as severe headache, high fever, or vomiting; or 8) suspicion of other intracranial complications. The primary clinician should provide the referral center with basic patient information and can request the results of medical examinations and treatment and also retransfer of the patient (Recommendation grade: B).
To diagnose complications of AOM, it is essential to determine the precise medical history over time. First, it is important to determine whether the disease is acute or chronic because the causative agent, medical treatment, and complications differ between these two conditions. History-taking should include details of the last time the patient had a medical examination of the ear, when and how the patient was treated in the event of previous AOM, whether antibiotics were taken for AOM, the time course and severity of present symptoms, and the results of tests, including tympanometry, audiometry, and radiological examinations. Concurrent severe vertigo implies labyrinthitis and the patient's alertness can change over time with intracranial complications. Changes in alertness are also important because alertness rapidly deteriorates within 1-2 days in cases of meningitis, but several weeks after disease onset with a brain tumor. Especially, intracranial complications such as headache, fever, vomiting, neck stiffness, convulsions, or changes in vision and alertness arising with disease progression should be considered in patients with severe otalgia without improvement after adequate treatment (47).
OME is defined as the presence of middle ear fluid without acute signs or symptoms. Acute signs and symptoms associated with OM should be identified as absent by history taking and physical examination. The presence of fluid in the middle ear can be determined by physical examination using electric otoscopy, pneumatic otoscopy, otoendoscopy, or otomicroscopy with support of tympanometry (Recommendation grade: A).
Each particular modality has advantages and drawbacks. Electric otoscopy is convenient and easy to apply but does not allow bimanual manipulation. Hence, examination of the tympanic membrane is difficult when a substantial amount of cerumen is present. The 2004 AAO guidelines recommended pneumatic otoscopy as the most sensitive tool for diagnosis of OME (13). This method enables examination of tympanic membrane mobility, but it has the drawback of being difficult to use. Correct occlusion is mandatory to guarantee a correct diagnosis. The greatest disadvantage of pneumatic otoscopy in Korea is that it is not widely used in primary clinical settings. Otoendoscopy provides a magnified view of the tympanic membrane, but it can induce traumatic injury of the tympanic membrane and the external auditory canal if not applied carefully. More importantly, the color and shape of the image can be distorted depending on the quality of the instruments used, including the endoscope, camera, and monitor. Otomicroscopy has been reported to be the most sensitive and specific approach, but the child's cooperation is necessary for proper examination, and it is not widely used in the primary care setting (49, 50). The guidelines recommend applying any of the available methods but taking each of their advantages and disadvantages into consideration.
Findings of the tympanic membrane that suggest middle ear fluid include air-fluid level, air bubbles, retraction, atelectasis, hyperemia, and change in color (amber, brownish, dark blue, etc.). Hyperemia of the tympanic membrane may be examined while the child is crying. Tympanometry can assist diagnosis of OME and is very useful when cooperation is lacking. OME can be suggested based on a type B or C tympanogram (Fig. 2).
Evaluation of hearing status in affected children is strongly recommended in these guidelines (Recommendation grade: A). Audiometric tests can be performed 1) at the time of diagnosis to measure hearing threshold, 2) any time during the follow-up period when presenting symptoms necessitate confirmation of hearing status, and 3) after a 3-month observation period for treatment planning. The appropriate method according to the developmental age of the particular child should be applied.
Generally, recommended tests for measuring hearing threshold for young children are behavioral observation audiometry for children under 6 months old, visual reinforcement audiometry for children 6-24 months old, and play audiometry for children 24-48 months old. Standard pure tone audiometry is usually applicable in children over 4 yr old (51). When cooperation is insufficient, the threshold of auditory brainstem response or distortion-product otoacoustic emission audiogram may be used to estimate hearing threshold, but cannot replace behavioral measurements.
The indications for hearing test recommended in these guidelines are highlighted to a greater extent than in other published guidelines (12, 13), a policy that reflects the easier accessibility and lower cost of audiometric tests in Korea than in other countries. With regard to hearing status as one of the most significant factors for medical decisions and parental concern, these guidelines adopted a positive policy on hearing tests, and so formulated recommendations according to which audiometry can be repeated whenever necessary.
Similar to other published guidelines, patients with medical conditions that warrant prompt and active intervention in OME and related hearing loss were defined as the high-risk group. This was to facilitate individualized intervention to prevent considerable negative impact on the development of language and other cognitive functions (12, 13).
Criteria to define children at high risk in these guidelines are: 1) sensorineural hearing loss independent of OME; 2) uncorrectable visual impairment; 3) Down's syndrome or craniofacial anomalies; 4) cleft palate; 5) autism spectrum disorder or pervasive developmental disorder; 6) suspected or diagnosed speech and language delay; and 7) the developmental or other cognitive impairments (Recommendation grade: B).
Children at high risk are those with factors that make them vulnerable to the negative influence of OME-driven hearing impairment with regard to education and development. In children with cleft palate, in whom the incidence and recurrence of OME have been reported to be elevated, OME-related hearing loss has been shown to be more frequent and more profound than in otherwise healthy children with OME (52-54).
In children with the aforementioned risk factors, their medical and developmental status should be carefully investigated, if necessary including consultation with related professionals such as an otolaryngologist, pediatrician, pediatric psychiatrist, speech therapist, audiologist, and physical therapist. Management of children at increased risk for developmental delay should include age-appropriate hearing testing and active intervention for associated hearing loss. Surgical intervention may be placed earlier and a hearing aid may be applied according to the hearing status either with or without surgical intervention. When a child is suspected to have language delay, prompt evaluation of language development is recommended to enable appropriate therapy designed for the specific problem of the child.
The guidelines recommend that clinicians manage children with OME according to the observation policy for at least 3 months from onset, unless the child has any of the following exceptional factors. Exceptional cases that warrant earlier intervention include 1) children with high-risk factors as listed above, 2) when an irreversible change of the tympanic membrane is anticipated, and 3) presenting signs and symptoms suggesting complications, such as sudden aggravation of hearing loss or dizziness. Further treatment should be planned according to the status of the tympanic membrane, hearing status, and developmental status of language after a 3-month observation period (Recommendation grade: A).
Current evidence regarding the efficacy of OME treatment discourages the use of medications, including antibiotics, oral and nasal steroids, antihistamines, and decongestants (55-59). The short-term benefits of antibiotics and antibiotic-steroid combination therapy have been reported, but the benefit becomes non-significant over long-term follow-up (58, 59). A randomized study performed in Korea also showed that there is no difference in the cure rate in children treated with antibiotics, antibiotics + steroid, antibiotics + antihistamine, and antibiotics + steroid + antihistamine compared to controls treated with only a mucolytic (60). Antihistamine or steroid use also showed no benefit for pediatric OME patients with nasal allergy (60).
In some cases, parents may be reluctant to agree to surgical intervention even in cases where early intervention is required, or may express anxiety over the lack of medication despite sufficient consultation. In such cases, antibiotic or antibiotic-steroid combination therapy can be administered for a short time (Recommendation grade: C). However, the observation policy suggested in these guidelines is not averse to medical therapy for symptoms associated with other concomitant diseases, such as sinusitis, pharyngitis, and adenotonsillitis. Children should be treated for all current problems with appropriate medical, surgical, and educational interventions.
The irreversible changes in the tympanic membrane that may accompany OME include retraction pocket, cholesteatoma, ossicular erosion, and adhesive OM. Immediate surgical intervention is generally recommended in children showing changes in the tympanic membrane during the 3-month observation period that may progress to any of the irreversible changes.
This observation policy is not intended to leave patients without intervention. To evaluate the status of the middle ear and the tympanic membrane, physicians may perform audiometric tests, such as impedance audiometry, tests for Eustachian tube function, and tests to measure hearing thresholds during the follow-up period. Hearing status of the affected child should be monitored carefully at the time of diagnosis of OME and during the observation period using age-appropriate means of audiometry. For children who show symptoms and signs of hearing impairment, information should be provided to the parent or caregiver with the goal of optimizing the listening environment at home and school. Autoinflation may be used in cooperative children during the observation period, as the risks and costs related to this maneuver are very low, although the evidence supporting the benefit of autoinflation is not robust (61). As stated above, the guidelines emphasize close monitoring of hearing and parental education in agreement with the concept of "active observation" and "watchful waiting" proposed in previous guidelines published in other countries (12, 13).
Surgical intervention for OME should be decided after considering the bilaterality of the disease, hearing status, duration of disease in the affected ear(s), parental preference, and effects on educational and developmental status of the child.
The decision for surgical intervention is made after 3 months of observation with evaluation of hearing. Exceptional cases for which surgical intervention should be considered earlier are 1) children at high risk and 2) children for whom changes in the tympanic membrane are anticipated to progress to irreversible changes (Recommendation grade: A). For otherwise healthy children, hearing status is a key factor determining whether surgery should be performed. In bilaterally affected cases, surgical intervention is recommended if hearing level in the better ear is 40-dB HL or above. If hearing level is between 20- and 40-dB HL, the guidelines recommend making a decision considering the medical/developmental status of child and parental preference (Recommendation grade: A). In unilaterally affected children, physicians may consider surgery, taking the duration of disease, hearing status, and parental preference into consideration (Recommendation grade: C).
In some cases, even though the hearing level meets the criteria for surgery, the surgical approach may not be possible for reasons such as other medical problems or lack of consent by the parent or caregiver. For these children, hearing status should be monitored at 1-3-month intervals, and clinicians may discuss the application of hearing aids and strategies to minimize the impact of hearing impairment. If an affected child shows a hearing threshold of 20-dB HL or better, physicians can offer a regular checkup for physical examination and audiometric surveillance to decide on further interventions when necessary.
It is generally agreed that surgical intervention is necessary when a child shows hearing loss of a moderate degree or worse and when the tympanic membrane is anticipated to develop irreversible changes. When OME persists over the 3-month observation period but the hearing threshold in the better ear is lower than the criterion demanding surgical intervention, the duration of disease is considered as the most crucial factor to determine whether surgical intervention should be performed. The potential negative impact of hearing impairment on language delay can be a concern over a prolonged period of observation (62, 63), but recent evidence from randomized prospective studies supports delayed intervention (64-66). In a series of prospective studies, children in a late-intervention group, who underwent surgery based on an examination conducted 9 months later in unilateral cases and 6 months later in bilateral cases, compared with an early intervention group, showed no difference in linguistic/social/educational status examined by repeated measurement over 11 yr (67-70). For a child with OME who does not meet the surgical criteria after the 3-month observation period, clinicians should discuss with the parent or caregiver whether to extend the observation period considering hearing threshold and developmental/social status of the child to minimize the risks and medical costs associated with surgery. Placement of a ventilation tube is the preferred method for surgical intervention as the initial procedure (refer the next section for details).
Ventilation tube insertion is the preferred initial procedure when a child becomes a surgical candidate. Adenoidectomy and/or tonsillectomy may be performed simultaneously if the status of the adenoid and the pharyngeal tonsil indicates these additional procedures. Adenoidectomy may be performed with ventilation tube insertion in recurrent cases for repeated surgical intervention (Recommendation grade: B).
Ventilation tube insertion is a safe procedure and improves hearing during placement of the tube, but it frequently produces abnormalities in the tympanic membrane such as tympanosclerosis and segmental atrophy (71-76). As the ventilation tube may not show long-term effects, surgical decisions should be made with the parents considering the risks and costs associated with the procedure.
Data from a recent meta-analysis and evidence from Korea support the benefit of adenoidectomy to reduce recurrence of OME, but the cost effectiveness of this procedure as the initial surgery is still debatable (77, 78). Adenoidectomy may be combined in the first surgery for OME, but the surgeon should consider the status of adenoidal pathology and cost effectiveness before making a decision.
Tonsillectomy has been reported to have no effect on OME and requires additional hospitalization with increased complication rates (78-80). Therefore, tonsillectomy may be performed if a distinct indication for the procedure exists concomitant with surgical procedures for OME, but should not be performed to treat OME.
After ventilation tube insertion, the guidelines recommend performing audiometric tests to confirm hearing recovery. In rare cases, hearing impairment may persist due to a hidden pathology that is independent of OME. Regular check-ups at 1-3-month intervals are recommended to examine tube-associated infection and extrusion of the tube. After extrusion of the ventilation tube, healing of the tympanic membrane and recurrence of OME should be evaluated.
When a clinician provides medical care for a child with OME, the guidelines recommend providing clear information about the etiology and natural course of the disease as well as treatment options to the patient and his/her parent or caregiver (Recommendation grade: B). It is good practice for medical professionals to provide not only verbal information but also written documents for patients and their family members. As OME may be recurrent or persistent during childhood until the function of the Eustachian tube develops, constant follow-up lasting from months to many years is necessary. Sufficient knowledge and appropriate understanding about the disease by the patient and the family is essential for a good doctor-patient relationship.
To facilitate continuing care, documentation of detailed history is recommended including details of the onset of OME, symptoms derived from OME, associated symptoms, past medical history, and presence of high-risk factors of the child (Recommendation grade: B). Primary clinicians may refer the patient to other medical centers, including higher referral centers for audiometric/developmental evaluation and surgical intervention. The primary clinician should provide the referred center with basic patient information and can request the results of medical examinations and treatment and also retransfer of the patient (Recommendation grade: B). Examples of documentation and referral forms are included as appendices in the full version available on the website.
No recommendations for complementary and alternative medicine for treatment of OME are made based on limited supporting data (Recommendation grade: D). After a literature search, we found four published papers in Korean regarding herbal medicine and acupuncture as treatments for OME. However, the sizes of these studies were very small, and the diagnostic criteria used were unclear. Therefore, the evidence levels were determined to be insufficient for inclusion in the development of these guidelines. Therefore, no recommendations are made regarding this subject.
These are the first guidelines for pediatric OM in Korea, and they represent an important and meaningful base on which to incorporate future revisions as necessary. These first guidelines call for regular nationwide surveys to improve scientific evidence on pediatric OM in Korea to be included in the next update. Future research topics should include better methods for diagnosis of pediatric OM and hearing assessment, better definition of children at high risk, the causative bacteria for AOM and changes in multidrug-resistant bacteria, long-term follow-up studies to evaluate developmental status according to treatment options for OME, cost-effectiveness of various treatments, and both medical and socioeconomic effects of alternative medicine for pediatric OM.
The recommendations are not intended to restrain professional judgment. Rather, they may be considered as professional advice on individual clinical circumstances. Guidelines represent the consensus of a team of experienced clinicians addressing the scientific evidence for a particular topic. Due to differences in physical characteristics and circumstances among patients, individual clinical characteristics and environmental factors should be given priority, and the clinical experience and judgment of attending physicians must be respected. In practice, all clinicians should always act and decide in a manner that they believe will best serve the individual patient's well-being regardless of the recommendations. Clinicians should actually select a treatment based on their professional knowledge and experience, along with the preference and values of the patient and parents/caregivers.
Figures and Tables
Fig. 2
Algorithm for management of pediatric otitis media with effusion (OME) in Korea. dB, decibel; HL, hearing level.

ACKNOWLEDGMENTS
This study received administrative support from Korean Otologic Society, Korean Pediatric Society, Korean Academy of Family Medicine, and Korean Society of Otorhinolaryngologic Clinicians. Authors are grateful to Prof. Eui-Kyung Goh, Prof. Keehyun Park, Prof. Myung-Hyun Chung, Prof. Sun O Chang, and Dr. Jeong-Joon Lee for careful comments while formulating recommendations and Korean Medical Guideline Information Center (KMGIC) for providing methodological support.
References
1. Korea Centers for Disease Control and Prevention. Korea health statistics 2009: Korea national health and nutrition examination survey (KNHANES IV-3). accessed on 20 February 2012. Available at http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/index.html.
2. Terris MH, Magit AE, Davidson TM. Otitis media with effusion in infants and children. Primary care concerns addressed from an otolaryngologist's perspective. Postgrad Med. 1995. 97:137–138. 143–144. 147
3. Kim CS, Jung HW, Yoo KY. Prevalence of otitis media and allied diseases in Korea-results of a nation-wide survey, 1991. J Korean Med Sci. 1993. 8:34–40.
4. Kim JG, Sohn YT. Prevalence of otitis media with effusion in kindergarten children in Taegu area. Korean J Otolaryngol-Head Neck Surg. 1995. 38:1695–1703.
5. Yeom MS, Lee SY, Lee HJ, Jeong KY. Prevalence of silent otitis media with effusion in preschool children in Kunsan city. J Korean Acad Fam Med. 1997. 18:46–52.
6. Pyo SY, Hong NP, Choo JH, Ahn HY, Cha CI, Jo JH. The prevalence of otitis media with effusion among kindergarten and elementary school children in Song Buk, Seoul, Korea and risk factors. Korean J Otolaryngol-Head Neck Surg. 2000. 43:1158–1165.
7. Chae SW, Hwang KS, Suh HK, Lim HH, Jung HH, Hwang SJ. The point prevalence of otitis media with effusion among kindergarten and elementary school children in Ansan area. Korean J Otolaryngol-Head Neck Surg. 1999. 42:700–703.
8. Chang KH, Park SN, Kim HJ, Yoon HR. A prevalence study of otitis media with effusion in kindergarten children in Puchun. Korean J Otolaryngol-Head Neck Surg. 1997. 40:374–381.
9. Facione N. Quality of life issues in chronic otitis media with effusion: parameters for future study. Int J Pediatr Otorhinolaryngol. 1991. 22:167–179.
10. Kim SH, Hong HJ, Kim HJ. Effect of ventilation tube insertion on the quality of life. Korean J Otolaryngol-Head Neck Surg. 2003. 46:296–301.
11. American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004. 113:1451–1465.
12. National Collaborating Centre for Women's Health and Children's Health. Surgical management of otitis media with effusion in children. 2008. London: RCOG Press;1–92.
13. Rosenfeld RM, Culpepper L, Doyle KJ, Grundfast KM, Hoberman A, Kenna MA, Lieberthal AS, Mahoney M, Wahl RA, Woods CR Jr, et al. Clinical practice guideline: otitis media with effusion. Otolaryngol Head Neck Surg. 2004. 130:S95–S118.
14. Subcommittee of Clinical Practice Guideline for Diagnosis and Management of Acute Otitis Media in Children (Japan Otological Society, Japan Society for Pediatric Otorhinolaryngology, Japan Society for Infectious Diseases in Otolaryngology). Clinical practice guidelines for the diagnosis and management of acute otitis media (AOM) in children in Japan. Auris Nasus Larynx. 2012. 39:1–8.
15. Marchisio P, Bellussi L, Di Mauro G, Doria M, Felisati G, Longhi R, Novelli A, Speciale A, Mansi N, Principi N. Acute otitis media: from diagnosis to prevention. summary of the Italian guideline. Int J Pediatr Otorhinolaryngol. 2010. 74:1209–1216.
16. Choi EH. Emergence of macrolide resistance and clinical use of macrolide antimicrobials in children. Korean J Pediatr. 2008. 51:1031–1037.
17. Kang JH, Kim SM, Kim JH, Hur JK, Lee KY, Shin YK, Park SE, Ma SH, Hong YJ. Penicillin resistant distribution and in-vitro susceptibility of oral antibiotics against Streptococcus pneumoniae, isolated from pediatric patients with community-acquired respiratory infections in Korea. Korean J Pediatr. 2005. 48:40–47.
18. Harabuchi Y, Kodama H, Faden H. Outcome of acute otitis media and its relation to clinical features and nasopharyngeal colonization at the time of diagnosis. Acta Otolaryngol. 2001. 121:908–914.
19. Hotomi M, Yamanaka N, Shimada J, Ikeda Y, Faden H. Factors associated with clinical outcomes in acute otitis media. Ann Otol Rhinol Laryngol. 2004. 113:846–852.
20. Hotomi M, Yamanaka N, Samukawa T, Suzumot M, Sakai A, Shimada J, Ikeda Y, Faden H. Treatment and outcome of severe and non-severe acute otitis media. Eur J Pediatr. 2005. 164:3–8.
21. Jang CH, Kim YH. Clinical usefulness of temperature of tympanic membrane in diagnosisg unilateral acute suppurative otitis media. Korean J Otolaryngol-Head Neck Surg. 2000. 43:715–718.
22. Revai K, Patel JA, Grady JJ, Chonmaitree T. Tympanometric findings in young children during upper respiratory tract infections with and without acute otitis media. Pediatr Infect Dis J. 2008. 27:292–295.
23. Lee SW, Park SH, Chung YY, Oh CH. Tympanometric changes following acute otitis media in children. Korean J Audiol. 1999. 3:123–130.
24. Rosenfeld RM, Kay D. Natural history of untreated otitis media. Laryngoscope. 2003. 113:1645–1657.
25. Damoiseaux RA, van Balen FA, Hoes AW, Verheij TJ, de Melker RA. Primary care based randomised, double blind trial of amoxicillin versus placebo for acute otitis media in children aged under 2 years. BMJ. 2000. 320:350–354.
26. Siegel RM, Kiely M, Bien JP, Joseph EC, Davis JB, Mendel SG, Pestian JP, DeWitt TG. Treatment of otitis media with observation and a safety-net antibiotic prescription. Pediatrics. 2003. 112:527–531.
27. Babin E, Lemarchand V, Moreau S, Goullet de Rugy M, Valdazo A, Bequignon A. Failure of antibiotic therapy in acute otitis media. J Laryngol Otol. 2003. 117:173–176.
28. Glasziou PP, Del Mar CB, Sanders SL, Hayem M. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev. 2004. CD000219.
29. Spiro DM, Tay KY, Arnold DH, Dziura JD, Baker MD, Shapiro ED. Wait-and-see prescription for the treatment of acute otitis media: a randomized controlled trial. JAMA. 2006. 296:1235–1241.
30. Spiro DM, Arnold DH. The concept and practice of a wait-and-see approach to acute otitis media. Curr Opin Pediatr. 2008. 20:72–78.
31. Brook I, Gober AE. Effect of amoxicillin and co-amoxiclav on the aerobic and anaerobic nasopharyngeal flora. J Antimicrob Chemother. 2002. 49:689–692.
32. Park SE. Antibiotics therapy of respiratory infection in outpatients department. Korean J Pediatr Infect Dis. 2003. 10:61–70.
33. Pichichero ME, Casey JR. Diagnostic inaccuracy and subject exclusions render placebo and observational studies of acute otitis media inconclusive. Pediatr Infect Dis J. 2008. 27:958–962.
34. Kozyrskyj AL, Hildes-Ripstein GE, Longstaffe SE, Wincott JL, Sitar DS, Klassen TP, Moffatt ME. Short course antibiotics for acute otitis media. Cochrane Database Syst Rev. 2000. CD001095.
35. Ovetchkine P, Cohen R. Shortened course of antibacterial therapy for acute otitis media. Paediatr Drugs. 2003. 5:133–140.
36. Paik JY, Choi JH, Cho EY, Oh CE, Lee J, Choi EH, Lee HJ. Antibiotics susceptability of Streptococcus pneumoniae isolated from pharynx in healthy Korean children and choice of proper empirical oral antibiotics using pharmacokinetics/pharmacodynamics model. Korean J Pediatr Infect Dis. 2011. 18:109–116.
37. Ghaffar F, Muniz LS, Katz K, Reynolds J, Smith JL, Davis P, Friedland IR, McCracken GH Jr. Effects of amoxicillin/clavulanate or azithromycin on nasopharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae in children with acute otitis media. Clin Infect Dis. 2000. 31:875–880.
38. Ghaffar F, Muniz LS, Katz K, Smith JL, Shouse T, Davis P, McCracken GH Jr. Effects of large dosages of amoxicillin/clavulanate or azithromycin on nasopharyngeal carriage of Streptococcus pneumoniae, Haemophilus influenzae, nonpneumococcal α-hemolytic streptococci, and Staphylococcus aureus in children with acute otitis media. Clin Infect Dis. 2002. 34:1301–1309.
39. Coleman C, Moore M. Decongestants and antihistamines for acute otitis media in children. Cochrane Database Syst Rev. 2008. CD001727.
40. Asher E, Dagan R, Greenberg D, Givon-Lavi N, Libson S, Porat N, Leiberman A, Leibovitz E. Persistence of pathogens despite clinical improvement in antibiotic-treated acute otitis media is associated with clinical and bacteriologic relapse. Pediatr Infect Dis J. 2008. 27:296–301.
41. Dagan R, Barkai G, Givon-Lavi N, Sharf AZ, Vardy D, Cohen T, Lipsitch M, Greenberg D. Seasonality of antibiotic-resistant Streptococcus pneumoniae that causes acute otitis media: a clue for an antibiotic-restriction policy? J Infect Dis. 2008. 197:1094–1102.
42. Lubianca Neto JF, Hemb L, Silva DBE. Systematic literature review of modifiable risk factors for recurrent acute otitis media in childhood. J Pediatr (Rio J). 2006. 82:87–96.
43. Pavia M, Bianco A, Nobile CGA, Marinelli P, Angelillo IF. Efficacy of pneumococcal vaccination in children younger than 24 months: a meta-analysis. Pediatrics. 2009. 123:e1103–e1110.
44. Jang GC, Shin KM, Yong DE, Lee KW, Kim DS. Changing patterns of antibiotic-resistant rates and clinical features in pneumococcal infections. Korean J Pediatr Infect Dis. 2003. 10:81–86.
45. Kim SM, Hur JK, Lee KY, Shin YK, Park SE, Ma SH, Min AY, Kang JH. Epidemiological study of pneumococcal nasal carriage and serotypes among Korean children. Korean J Pediatr. 2004. 47:611–616.
46. Straetemans M, Sanders EA, Veenhoven RH, Schilder AG, Damoiseaux RA, Zielhuis GA. Review of randomized controlled trials on pneumococcal vaccination for prevention of otitis media. Pediatr Infect Dis J. 2003. 22:515–524.
47. Thorne MC, Chewaproug L, Elden LM. Suppurative complications of acute otitis media: changes in frequency over time. Arch Otolaryngol Head Neck Surg. 2009. 135:638–641.
48. Karkos PD, Leong SC, Arya AK, Papouliakos SM, Apostolidou MT, Issing WJ. "Complementary ENT": a systematic review of commonly used supplements. J Laryngol Otol. 2007. 121:779–782.
49. Lee DH. How to improve the accuracy of diagnosing otitis media with effusion in a pediatric population. Int J Pediatr Otorhinolaryngol. 2010. 74:151–153.
50. Young DE, Ten Cate WJ, Ahmad Z, Morton RP. The accuracy of otomicroscopy for the diagnosis of paediatric middle ear effusions. Int J Pediatr Otorhinolaryngol. 2009. 73:825–828.
51. Diefendorf A. Katz J, editor. Detection and assessment of hearling loss in infants and children. Handbook of clinical audiology. 2002. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins;469–480.
52. Valtonen H, Dietz A, Qvarnberg Y. Long-term clinical, audiologic, and radiologic outcomes in palate cleft children treated with early tympanostomy for otitis media with effusion: a controlled prospective study. Laryngoscope. 2005. 115:1512–1516.
53. Lee HK, Koh KM, Kim KR, Park CW, Ahn KS, Uhm KI. Evaluation of otitis media with effusion in cleft palate patients. Korean J Otolaryngol-Head Neck Surg. 1995. 38:230–235.
54. Flynn T, Möller C, Jönsson R, Lohmander A. The high prevalence of otitis media with effusion in children with cleft lip and palate as compared to children without clefts. Int J Pediatr Otorhinolaryngol. 2009. 73:1441–1446.
55. Griffin G, Flynn CA. Antihistamines and/or decongestants for otitis media with effusion (OME) in children. Cochrane Database Syst Rev. 2011. CD003423.
56. Williamson I, Benge S, Barton S, Petrou S, Letley L, Fasey N, Abangma G, Dakin H, Little P. A double-blind randomised placebo-controlled trial of topical intranasal corticosteroids in 4- to 11-year-old children with persistent bilateral otitis media with effusion in primary care. Health Technol Assess. 2009. 13:1–144.
57. Petrou S, Dakin H, Abangma G, Benge S, Williamson I. Cost-utility analysis of topical intranasal steroids for otitis media with effusion based on evidence from the GNOME trial. Value in Health. 2010. 13:543–551.
58. Mandel EM, Casselbrant ML, Rockette HE, Fireman P, Kurs-Lasky M, Bluestone CD. Systemic steroid for chronic otitis media with effusion in children. Pediatrics. 2002. 110:1071–1080.
59. Simpson SA, Lewis R, van der Voort J, Butler CC. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2011. CD001935.
60. Choung YH, Shin YR, Choi SJ, Park K, Park HY, Lee JB, Han DH, Kahng H. Management for the children with otitis media with effusion in the tertiary hospital. Clin Exp Otorhinolaryngol. 2008. 1:201–205.
61. Perera R, Haynes J, Glasziou P, Heneghan CJ. Autoinflation for hearing loss associated with otitis media with effusion. Cochrane Database Syst Rev. 2006. CD006285.
62. Maw R, Wilks J, Harvey I, Peters TJ, Golding J. Early surgery compared with watchful waiting for glue ear and effect on language development in preschool children: a randomised trial. Lancet. 1999. 353:960–963.
63. Rach GH, Zielhuis GA, van Baarle PW, van den Broek P. The effect of treatment with ventilating tubes on language development in preschool children with otitis media with effusion. Clin Otolaryngol Allied Sci. 1991. 16:128–132.
64. Stenstrom R, Pless IB, Bernard P. Hearing thresholds and tympanic membrane sequelae in children managed medically or surgically for otitis media with effusion. Arch Pediatr Adolesc Med. 2005. 159:1151–1156.
65. Lous J. Which children would benefit most from tympanostomy tubes (grommets)? A personal evidence-based review. Int J Pediatr Otorhinolaryngol. 2008. 72:731–736.
66. Rovers MM, Straatman H, Ingels K, van der Wilt GJ, van den Broek P, Zielhuis GA. The effect of ventilation tubes on language development in infants with otitis media with effusion: a randomized trial. Pediatrics. 2000. 106:e42.
67. Paradise JL, Feldman HM, Campbell TF, Dollaghan CA, Colborn DK, Bernard BS, Rockette HE, Janosky JE, Pitcairn DL, Sabo DL, et al. Effect of early or delayed insertion of tympanostomy tubes for persistent otitis media on developmental outcomes at the age of three years. N Engl J Med. 2001. 344:1179–1187.
68. Paradise JL, Dollaghan CA, Campbell TF, Feldman HM, Bernard BS, Colborn DK, Rockette HE, Janosky JE, Pitcairn DL, Kurs-Lasky M, et al. Otitis media and tympanostomy tube insertion during the first three years of life: developmental outcomes at the age of four years. Pediatrics. 2003. 112:265–277.
69. Paradise JL, Campbell TF, Dollaghan CA, Feldman HM, Bernard BS, Colborn DK, Rockette HE, Janosky JE, Pitcairn DL, Kurs-Lasky M. Developmental outcomes after early or delayed insertion of tympanostomy tubes. N Engl J Med. 2005. 353:576–586.
70. Paradise JL, Feldman HM, Campbell TF, Dollaghan CA, Rockette HE, Pitcairn DL, Smith CG, Colborn DK, Bernard BS, Kurs-Lasky M, et al. Tympanostomy tubes and developmental outcomes at 9 to 11 years of age. N Engl J Med. 2007. 356:248–261.
71. Browning GG, Rovers MM, Williamson I, Lous J, Burton MJ. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2010. CD001801.
72. Valtonen HJ, Qvarnberg YH, Nuutinen J. Otological and audiological outcomes five years after tympanostomy in early childhood. Laryngoscope. 2002. 112:669–675.
73. Valtonen H, Tuomilehto H, Qvarnberg Y, Nuutinen J. A 14-year prospective follow-up study of children treated early in life with tympanostomy tubes: part 1: clinical outcomes. Arch Otolaryngol Head Neck Surg. 2005. 131:293–298.
74. Valtonen H, Tuomilehto H, Qvarnberg Y, Nuutinen J. A 14-year prospective follow-up study of children treated early in life with tympanostomy tubes: part 2: hearing outcomes. Arch Otolaryngol Head Neck Surg. 2005. 131:299–303.
75. Cayé-Thomasen P, Stangerup SE, Jørgensen G, Drozdziewic D, Bonding P, Tos M. Myringotomy versus ventilation tubes in secretory otitis media: eardrum pathology, hearing, and eustachian tube function 25 years after treatment. Otol Neurotol. 2008. 29:649–657.
76. Park CW, Park IB, Choi JS, Jeong YG, Ahn KS. Factors that affect the development of tympanosclerosis after ventilation tube insertion. Korean J Otolaryngol-Head Neck Surg. 2000. 43:15–19.
77. van den Aardweg MT, Schilder AG, Herkert E, Boonacker CW, Rovers MM. Adenoidectomy for otitis media in children. Cochrane Database Syst Rev. 2010. CD007810.
78. Jung MK, Yeo SW, Lee IR, Park JW, Nam JS. Efficacy of adenoidectomy in preventing recurrence of otitis media with effusion. Korean J Otolaryngol-Head Neck Surg. 2004. 47:293–298.
79. Paradise JL, Bluestone CD, Colborn DK, Bernard BS, Smith CG, Rockette HE, Kurs-Lasky M. Adenoidectomy and adenotonsillectomy for recurrent acute otitis media: parallel randomized clinical trials in children not previously treated with tympanostomy tubes. JAMA. 1999. 282:945–953.
80. Kadhim AL, Spilsbury K, Semmens JB, Coates HL, Lannigan FJ. Adenoidectomy for middle ear effusion: a study of 50,000 children over 24 years. Laryngoscope. 2007. 117:427–433.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download