Abstract
Thyrotoxic periodic paralysis (TPP) is a rare manifestation of hyperthyroidism characterized by muscle weakness and hypokalemia. All ethnicities can be affected, but TPP typically presents in men of Asian descent. The most common cause of TPP in thyrotoxicosis is Graves' disease. However, TPP can occur with any form of thyrotoxicosis. Up to our knowledge, very few cases ever reported the relationship between TPP and painless thyroiditis. We herein report a 25-yr-old Korean man who suffered from flaccid paralysis of the lower extremities and numbness of hands. The patient was subsequently diagnosed as having TPP associated with transient thyrotoxicosis due to painless thyroiditis. The paralytic attack did not recur after improving the thyroid function. Therefore, it is necessary that early diagnosis of TPP due to transient thyrotoxicosis is made to administer definite treatment and prevent recurrent paralysis.
Thyrotoxic periodic paralysis (TPP) is a rare disease characterized by episodes of muscle paralysis associated with hypokalemia and thyrotoxicosis. Attack of TPP is characterized by acute and reversible episodes of flaccid muscle paralysis affecting proximal more severely than distal muscle. The severity of the episodes ranges from mild weakness to complete flaccid paralysis. TPP is most common in Asian populations, with an incidence of approximately 2% in patients with thyrotoxicosis of any cause, although it can occur in other ethnic group as well. The most common cause of TPP in thyrotoxicosis is Graves' disease, although TPP can occur with any form of thyrotoxicosis (1, 2). It is less commonly associated with thyroiditis (3, 4), toxic adenoma (5), T4 ingestion and iodine excess (6). However, very few cases such as this one have been reported as far as we know that thyrotoxic periodic paralysis associated with painless thyroiditis. Here we report a case of TPP associated with transient thyrotoxicosis due to painless thyroiditis and review TPP associated with the other thyrotoxicosis except Graves' disease from the relative literature.
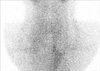
A 25-yr-old Korean man was admitted to Pusan National University Hospital, Busan, Korea, on May 26, 2011, complaining flaccid paralysis of the lower extremities and numbness of hands. On arrival at the emergency room, his blood pressure was 110/70 mmHg, and the heart rate was 72 beats/min. He was alert with a respiratory rate of 18 breaths/min and a body temperature of 36.5℃. No heat intolerance, weight loss, changes in bowel habits, and other symptoms of hyperthyroidism were reported. On physical examination, he had a thyroid of normal size and consistency. Auscultation of the thyroid revealed no bruit. No exophthalmos or skin change was present. The lower limbs had flaccid paralysis with intact sensory function. No respiratory or visual difficulties were detected. He had no significant medical history and had received no medication; his family history was negative for familial hypokalemic periodic paralysis or thyroid disease. Laboratory tests revealed the following serum metabolite levels ; on the day of admission, sodium 143.6 mEq/L, potassium 2.42 mEq/L, chloride 104.7 mEq/L, creatinine 0.6 mg/dL, calcium 9.4 mg/dL and phosphorus 3.4 mg/dL. Arterial blood gas analysis showed arterial pH 7.408 mmHg, PO2 74.8 mmHg, PCO2 35.7 mmHg and bicarbonate 23.7 mM/L. A thyroid function test showed a thyroid-stimulating hormone level of 0.00 µIU/mL (normal: 0.3-5.0 µIU/mL), a T3 level of 205.1 ng/dL (normal: 80-170 ng/dL) and a free T4 level of 2.38 ng/dL (normal: 0.75-2.00 ng/dL). Thyroid autoantibodies showed a prominent elevated thyroglobulin antibody 93.40 U/mL (normal: 0-60 U/mL) and thyroid peroxidase antibody 642.82 U/mL (normal: 0-60 U/mL), but the level of TSH receptor antibody (by human TRAK RIA kit, BRAHMS, Hennigsdorf, Germany) was 0.37 IU/L (normal: 0-1.5 IU/L). An ultrasound revealed a normal sized thyroid gland, and neither nodules nor lymphadenopathy was detected (Fig. 1). A thyroid scan with Tc-99m revealed little or no uptake in the thyroid area compatible with thyroiditis (Fig. 2). Finally, we diagnosed this patient as thyrotoxic periodic paralysis associated with transient thyrotoxicosis due to painless thyroiditis.
He was given 40 mEq/L of intravenous potassium chloride in the emergency department and was then started on a normal saline infusion with 20 mEq/L of potassium chloride. He was also prescribed on an oral potassium and propranolol, which resulted in resolution of his lower extremity paralysis. On the second day of admission, his serum potassium level increased to 4.33 mEq/L. There was clinical improvement, concomitant with a progressive normalization of serum electrolytes. Complete remission of symptoms was obtained in 24 hr. Upon discharge, the patient had completely recovered his neuromuscular functions and serial measurement of his serum potassium level in the hospital remained within normal limits without oral potassium supplements.
He did not experience a hypokalemic paralytic attack during the subsequent 2 months. After 2 months of follow-up, thyroid function test was re-checked on outpatient department. The thyroid-stimulating hormone level was 11.11 µIU/mL (normal: 0.3-5.0 µIU/mL), a T3 level was 89.8 ng/dL (normal: 80-170 ng/dL), and a free T4 was 1.19 µg/dL (normal: 0.75-2.00 µg/dL); follow-up thyroid function survey was compatible with subclinical hypothyroidism. However, he remained symptom free.
In this article, we describe a patient suffering from transient thyrotoxicosis due to painless thyroiditis complicated with TPP. TPP is rare sporadic muscle disorder characterized by episodes of muscle paralysis associated with hypokalemia in some, but not all, thyrotoxic individuals. Patients have no symptoms between attacks, which resolve with treatment of thyrotoxicosis. TPP is the most frequent form of acquired acute flaccid paralysis in adults. It is considered a sporadic disease affecting most frequently Asian male suffering from hyperthyroidism in the second and third decade of their life (1). The incidence of TPP in Chinese and Japanese thyrotoxic patients has been reported at 1.8% and 1.9%, respectively (2), whereas in North Americans at 0.1%-0.2% (7). A male predominance has been widely described, with an overall male to female ratio ranging from 17:1 to 70:1 (2, 8).
Most patients with TPP have only mildly elevated serum thyroid hormone level. A previous study reported only 10% of patients with mild thyrotoxic symptoms (8). The hyperthyroidism may even be clinically silent. Some of 80% of TPP cases arise in the acute phase of hyperthyroidism, although they can be preceded by endocrine symptoms lasting 3 months to 12 months. Muscle paralysis may be the only symptom at first manifestation of hyperthyroidism, and it can appear with normal serum levels of the thyroid hormones. In the present case, serum free T4 and T3 were slightly elevated above normal range, and TSH was nondetectable at the time of admission.
Although the majority of cases of thyrotoxicosis associated with TPP are due to Graves' disease, TPP can appear with thyrotoxicosis of any origin. Patients with TPP have been reported with thyroiditis (3, 4), toxic adenoma (5), toxic nodular goiter (9), TSH-secreting pituitary adenoma (10, 11), ingestion of T4 or T3 (6, 12, 13), inadvertent iodine excess (14), subacute thyroiditis of de Quervain (15), amiodarone therapy (16), radiation thyroiditis with Graves' disease (17) and nutraceuticals containing triatricol (18).
Our case will have a significance, we think, as there have been very rare cases of TPP associated with transient thyrotoxicosis state of painless thyroiditis. Including the present case, we identified some cases of thyrotoxic periodic paralysis not related with Graves' disease in the English literature between 1981 and 2011 (Table 1). There were 11 men and a woman with the mean age (± SD) of 31.7 ± 6.8 yr. The mean level of serum potassium (± SD) was 1.97 ± 0.42 mEq/L. The most common causes of TPP except Graves' disease were thyroiditis and factitious thyrotoxicosis. Interestingly, in one patient, serious complication such as nearfatal ventricular tachycardia due to hypokalemia developed (3). Although the hallmark of TPP is hypokalemia, hypokalemia does not indicate a depletion of the total potassium pool but an increased influx of the ion to the intracellular compartment. The presenting serum potassium level is usually less than 3.0 mEq/L and can be as low as 1.1 mEq/L (1). Thus the serum potassium level and electrocardiogram should be checked in all patients with thyrotoxic periodic paralysis.
Although the pathogenesis of TPP remains unclear, recently many evidences indicate that TPP results from the combination of genetics, thyrotoxicosis and environment factors. Excessive levels of T3, androgen, carbohydrate-rich meals and rest following exercise could exert their effects by altering ion channel dynamics in the cell membranes of neuromuscular junctions; these factors converge to trigger paralysis in susceptible patients. In this etiopathogenic hypothesis, excessive T3 has a major role in revealing genetic defects in patients with TPP (19).
In typical TPP, hypokalemia is the consequence of a rapid and massive shift of potassium from the extracellular into the intracellular compartment, mainly into the muscles. Sodium, chloride, calcium, and potassium channels on cell membranes are responsible for membrane excitability and muscle contractions. Disruption of any of these cellular transport mechanisms - especially 3Na+/2K+ ATPase pump - may cause abnormalities in muscle contractibility, and paralysis. Thyroid hormones can increase 3Na+/2K+ ATPase activity in skeletal muscle, liver, and kidney to induce an influx of potassium into the intracellular space. In some data revealed an increased number as well as activity of the 3Na+/2K+ ATPase pump in patients with thyrotoxicosis. And patients with TPP had significantly higher pump activity than thyrotoxic patients without TPP. In all patients with hyperthyroidism, 3Na+/2K+ ATPase activity returns to normal with establishment of the euthyroid state.
In addition, insulin is another stimulator of 3Na+/2K+ ATPase pump as well. Insulin can play an important role for potassium shift in patients with TPP and insulin has been shown to enhance 3Na+/2K+ ATPase activity. The patients with TPP produce increased levels of insulin during attacks and have raised levels of basal insulin between attacks. The hyperinsulinemic response may explain the association of TPP with carbohydrate-rich meals and sweet snacks. Moreover, the patients with TPP had considerably lower insulin sensitivity than the patients who had thyrotoxicosis without periodic paralysis. Catecholamine can also increase 3Na+/2K+ ATPase activity in skeletal muscle. It may explain that nonselective beta-adrenergic blockers relieve paralytic symptom and prevent recurrent paralytic attacks. Androgens are also capable of increasing 3Na+/2K+ ATPase activity (19).
Taking these findings together, the possible mechanism of TPP have an underlying predisposition for activation of 3Na+/2K+ ATPase activity, either directly by thyroid hormone or indirectly via adrenergic stimulation, hyperinsulinemia and postexercise counter-regulatory mechanism. However, the reason for the ethnic difference remains undefined (1, 19).
Management of TPP includes correction of hypokalemia and treatment of the underlying hyperthyroid state. During periodic paralysis and marked hypokalemia, immediate supplement with potassium chloride is needed to prevent major cardiopulmonary complications. Generally, patients are given intravenous or oral potassium to hasten muscle recovery and prevent cardiopulmonary complications. However, there is a concern of rebound hyperkalemia due to release of potassium from the cells on recovery. Rebound hyperkalemia occurred in approximately 40% of patients of TPP, especially who received > 90 mEq/dL of potassium chloride within the first 24 hr. Therefore, lower doses of potassium chloride may be effective while lowering the patient's risk of hyperkalemia (1, 11).
To prevent attacks until euthyroid state is achieved, a useful therapy is the administration of a nonselective beta-adrenergic blocker like propranolol. It prevents the intracellular shift of potassium by inhibiting the hyperadrenergic stimulation of 3Na+/2K+ ATPase. The combination of low-dose of potassium chloride and nonselective beta-adrenergic blocker appears to be the treatment of choice for facilitating recovery and reducing rebound hyperkalemia. Because TPP does not recur once the patient is euthyroid, adequate control of hyperthyroidism is the mainstay of therapy. The cause for the hyperthyroidism should be evaluated, and patients should avoid precipitating factors including heavy carbohydrate intake, high salt diet and alcohol ingestion until thyrotoxicosis is under control (1, 19).
In conclusion, thyrotoxic periodic paralysis can occur in association with any of the cause of thyrotoxicosis. As we know, the most common cause of TPP in thyrotoxicosis is Graves' disease. Besides Graves' disease, patients with TPP have been reported with toxic nodular goiter, iodine-induced thyrotoxicosis, excessive thyroxine use, toxic thyroid adenoma, TSH-secreting pituitary adenoma, and so on. In this report, we describe a rare case of transient thyrotoxicosis due to painless thyroiditis discovered during investigations for the etiology of TPP. Although further studies are required in order to clarify the mechanism of TPP, this would be the important case of TPP as the manifestation of a transient thyrotoxicosis due to painless thyroiditis. Thus, painless thyroiditis should be kept in the differential diagnosis in any TPP patients to avoid delaying diagnosis and management.
Figures and Tables
Fig. 1
Ultrasound findings of the thyroid. (A) A gray scale showed a normal sized thyroid gland, and neither nodules nor lymphadenopathy was detected. (B) Power doppler image revealed decreased vascularity.

References
1. Kung AW. Clinical review. Thyrotoxic periodic paralysis: a diagnostic challenge. J Clin Endocrinol Metab. 2006. 91:2490–2495.
2. Okinaka S, Shizume K, Iino S, Watanabe A, Irie M, Noguchi A, Kuma S, Kuma K, Ito T. The association of periodic paralysis and hyperthyroidism in Japan. J Clin Endocrinol Metab. 1957. 17:1454–1459.
3. Lee JI, Sohn TS, Son HS, Oh SJ, Kwon HS, Chang SA, Cha BY, Son HY, Lee KW. Thyrotoxic periodic paralysis presenting as polymorphic ventricular tachycardia induced by painless thyroiditis. Thyroid. 2009. 19:1433–1434.
4. Tinker TD, Vannatta JB. Thyrotoxic hypokalemic periodic paralysis: report of four cases and review of the literature. J Okla State Med Assoc. 1987. 80:76–83.
5. Tagami T, Usui T, Shimatsu A, Naruse M. Toxic thyroid adenoma presenting as hypokalemic periodic paralysis. Endocr J. 2007. 54:797–803.
6. Chen YC, Fang JT, Chang CT, Chou HH. Thyrotoxic periodic paralysis in a patient abusing thyroxine for weight reduction. Ren Fail. 2001. 23:139–142.
7. Kelley DE, Gharib H, Kennedy FP, Duda RJ Jr, McManis PG. Thyrotoxic periodic paralysis. Report of 10 cases and review of electromyographic findings. Arch Intern Med. 1989. 149:2597–2600.
8. Ko GT, Chow CC, Yeung VT, Chan HH, Li JK, Cockram CS. Thyrotoxic periodic paralysis in a Chinese population. QJM. 1996. 89:463–468.
9. Ozaki H, Mori K, Nakagawa Y, Hoshikawa S, Ito S, Yosida K. Autonomously functioning thyroid nodule associated with thyrotoxic periodic paralysis. Endocr J. 2008. 55:113–119.
10. Hsu FS, Tsai WS, Chau T, Chen HH, Chen YC, Lin SH. Thyrotopin-secreting pituitary adenoma presenting as hypokalemic periodic paralysis. Am J Med Sci. 2003. 325:48–50.
11. Kiso Y, Yoshida K, Kaise K, Kaise N, Masuda T, Ando N, Kameyama M, Yamamoto M, Sakurada T, Yoshinaga K. A case of thyrotropin (TSH)-secreting tumor complicated by periodic paralysis. Jpn J Med. 1990. 29:399–404.
12. Hannon MJ, Behan LA, Agha A. Thyrotoxic periodic paralysis due to excessive L-thyroxine replacement in a Caucasian man. Ann Clin Biochem. 2009. 46:423–425.
13. Chou HK, Tsao YT, Lin SH. An unusual case of thyrotoxic periodic paralysis: triiodothyronine-containing weight reducing agents. Am J Med Sci. 2009. 337:71–73.
14. Ober KP, Hennessy JK. Jodbasedow and thyrotoxic periodic paralysis. Arch Intern Med. 1981. 141:1225–1227.
15. Piraino Neuenschwander P, Pumarino Carte H, Bidegain González F, Zura Jiménez ML, Ferreiro Merino F. Thyrotoxic hypokalemic periodic paralysis: 18 cases with different forms of thyrotoxicosis. Rev Clin Esp. 1995. 195:294–297.
16. Laroia ST, Zaw KM, Ganti AK, Newman W, Akinwande AO. Amiodarone-induced thyrotoxicosis presenting as hypokalemic periodic paralysis. South Med J. 2002. 95:1326–1328.
17. Akar S, Comlekci A, Birlik M, Onen F, Sari I, Gurler O, Bekis R, Akkoc N. Thyrotoxic periodic paralysis in a Turkish male: the recurrence of the attack after radioiodine treatment. Endocr J. 2005. 52:149–151.
18. Cohen-Lehman J, Charitou MM, Klein I. Tiratricol-induced periodic paralysis: a review of nutraceuticals affecting thyroid function. Endocr Pract. 2011. 17:610–615.
19. Maciel RM, Lindsey SC, Dias da Silva MR. Novel etiopathophysiological aspects of thyrotoxic periodic paralysis. Nat Rev Endocrinol. 2011. 7:657–667.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download