Abstract
We assessed whether the presence of juxtapapillary duodenal diverticula (JPDD) risks biliary stone disease and recurrence. In total, 695 patients who underwent ERCP were divided into two groups: biliary stone disease (group I, n = 523) and non-stone biliary diseases (group II, n = 172). Additionally, for a control group (group III), 80 age-matched healthy subjects underwent side-view duodenoscopy. In group I, rates of post-ERCP pancreatitis, cannulation failure, and disease recurrence in two-year follow up were compared according to the presence of JPDD. In results, the incidence of JPDD in group I (42.4%) was significantly higher than in group II (16.3%) and III (18.8%). The frequencies of JPDD were increased with age in all groups, and reached statistical significance in group I. In group I, rates of post-ERCP pancreatitis were significantly higher in patients with JPDD (18.5%) compared to JPDD negative (12.6%). The cannulation failure rate was also higher in patients with JPDD (9.9%) compared to JPDD negative (5.3%). Recurrence rate was higher in patients with JPDD (25.3%) compared to JPDD negative (9.2%). In conclusion, JPDD develops with aging and risks biliary stone formation. JPDD also seems to be associated with post-ERCP pancreatitis, cannulation failure and biliary stone recurrence.
Duodenal diverticula are bulging pouch-like herniations in the duodenal wall, and are typically found incidentally during esophagogastroduodenoscopy (EGD). Diverticula located near the major duodenal papilla are termed juxtapapillary duodenal diverticula (JPDD) (1). Such lesions are believed to be primarily acquired during the later decades of life, with the peak incidence occurring in individuals between 50 and 60 yr of age (2-6). Current estimates place the overall prevalence of JPDD from 12% to 21%, depending on the method of identification (1). However, such statistics were primarily culled from studies of patients with biliary stones or other biliary tract diseases and did not include healthy individuals. Although JPDD does not classically provoke symptoms by itself, some data have suggested an association between JPDD, bile duct stones, and increased incidence of complications and cannulation failures after endoscopic retrograde cholangiopancreatography (ERCP) (7-10).
Although several studies have correlated JPDD with biliary stone diseases, it has not yet been determined whether JPDD acts as an independent risk factor for biliary stone formation. Furthermore, the specific influence of JPDD on the risks of cannulation failure, other ERCP-associated complications (i.e., pancreatitis, and the overall success and recurrence rates were significantly different between studies.
The current study was designed to compare the relative incidences of JPDD between a large group of biliary stone disease patients and a second group of healthy age-matched controls. Secondarily, JPDD frequency was also stratified by type and association with biliary stone disease. Additional endpoints - including biliary stone disease complications (i.e., post-ERCP pancreatitis) and rates of cannulation failure and recurrence - were also tracked and evaluated.
In total, 695 patients were enrolled in the present study, all of whom underwent ERCP at Chonbuk National University Medical School in Jeonju, Korea between January 2006 and November 2007. In order to assess the influence of JPDD on biliary stone disease, patients were divided into a biliary stone disease group and a non-stone biliary disease group. The biliary stone disease group (group I, n = 523) included patients with common bile duct (CBD) stones and gallbladder (GB) stones with CBD sludge requiring ERCP. Patients showing definite whitish cholesterol stones in CBD which suggest migration from the GB were excluded. The non-stone biliary disease group (group II, n = 172) included cases of congenital anomalies (such as anomalous union of pancreaticobiliary duct [AUPBD] and choledochal cysts), biliary tract malignancies, non-biliary pancreatitis, intraductal papillary mucinous neoplasm (IPMN), and stone-unrelated biliary strictures. The control group (group III) was comprised of 80 healthy, age-matched peoples who underwent additional side-view duodenoscopy during a routine EGD for health screening purposes. None of these individuals had any GB or CBD stones. JPDD type was defined by proximity to the major duodenal papilla: diverticula with the papilla in the diverticular space (type 1), diverticula with the papilla on the diverticular margin (type 2), and papilla-adjacent diverticula (type 3) (Fig. 1) (11). CBD diameters were also measured at mid-CBD level of ERCP cholangiography in group I.
JPDD frequency and type were determined in all groups. Additional clinical data - including age, gender, CBD diameter, JPDD type, post-ERCP pancreatitis incidence, and cannulation failure rate - were also recorded and correlated with JPDD incidence. For our purposes, patients were determined to have post-ERCP pancreatitis if they presented with post-procedural abdominal pain lasting for longer than 24 hr, and had an associated elevation of serum amylase level at least three times greater than normal value (12). To evaluate the effect of JPDD on biliary stone recurrence, all biliary stone patients were followed for two years using clinical symptoms, laboratory findings, and varied imaging studies including ultrasonography (US) and abdominal computed tomography (CT). Here, biliary stone disease recurrence was defined as cases of new onset, imaging-proven biliary stones requiring hospitalization for ERCP. For the evaluation stone recurrence, the patient group should be classified into subgroups according to the techniques such as endoscopic sphincterotomy (EST) and endoscopic papillary balloon dilatation (EPBD). However, the influence of stone removal techniques were not considered in this study because of two techniques were applied together not infrequently with random manner.
In total, 250 of 695 patients (36.0%) were diagnosed to have JPDD by ERCP. Of these patients, 222 were in group I (n = 523; 42.4%), and 28 were in group II (n = 172; 16.3%). Additionally 15 individuals in group III (n = 80; 18.8%) were found to have JPDD. There was no significant age difference between groups. Overall, rates of JPDD were significantly higher in group I than in group II (P = 0.000) (Table 1). These results strongly suggest an association between JPDD and biliary stone disease. However, JPDD rates were not different between men and women in these data.
JPDD frequency also was observed to increase with age in all groups, with this relationship reaching statistical significance in groups I and II (P < 0.001) (Table 2). The relative prevalence of JPDD was found to be 13.5% in patients under the age of 50 yr, and 44.9% in patients between the ages of 70 to 79 yr. In the biliary stone disease group (group I), the mean age of patients with JPDD (69.58 ± 10.83) was significantly higher (7 yr older) than their counterparts lacking JPDD (62.26 ± 15.00) (P < 0.001). Although CBD diameters were relatively larger in JPDD positive patients (13.41 ± 4.12 mm) than the group without JPDD (12.80 ± 5.23 mm), this finding failed to reach statistical significance (P = 0.181). In combination, these results suggest that JPDD develop with aging and are associated with bile stasis and biliary stone formation.
The relative frequency of JPDD was further stratified according to subtype: 12.5% of JPDD were classified as type I, 43% as type 2, and 44.5% as type 3 (Table 3). Although the distribution of JPDD subtype by group was not significantly different between groups I and II (P = 0.257), type 1 JPDD were relatively more common (14.4%) in group I than in either group II (3.6%) or III (0%) (P = 0.142). These results suggest that possibility of statistical significance of type 1 JPDD as a greater risk of predisposition to biliary stone formation if the study was performed in more number of patients.
Of the 523 patients in group I, 79 (15.1%) suffered from post-ERCP pancreatitis. When stratified by JPDD prevalence, 18.5% of patients with JPDD developed post-ERCP pancreatitis, a significantly higher rate than those without JPDD (12.6%) (P = 0.043). The overall rate of CBD cannulation failure was also significantly higher in patients with JPDD (9.9%) when compared those without JPDD (5.3%) (P = 0.034). During the 2-yr follow up period, 16.1% of all patients experienced at least one episode of recurrent biliary stone disease. Specifically, recurrence rates were significantly higher in patients with JPDD (22.5%) when compared to JPDD-lacking individuals (11.3%) (P = 0.002). The mean time to recurrence and the mean number of recurrences did not vary significantly by JPDD status (Table 4). According to the subtype of JPDD, recurrence rate were 37.5% (12/32) in type 1, 31.3% (31/99) in type 2, 31.1% (28/90) in type 3, and it was not statistically different (P = 0.783). Post-ERCP pancreatitis rates were also not different according to the JPDD subtypes as follows; 9.4% (3/32) in type 1, 20.2% (20/99) in type 2 and 19.8% (18/91) in type 3 (P = 0.360). However, cannulation failure rate was higher in type 1 (7/32, 21.9%) than type 2 (7/99, 7.1%) and type 3 (8/91, 8.8%) (P = 0.039).
Of the 523 patients in group I, 207 (39.6%) underwent cholecystectomy to prevent cholecystitis and 316 (60.4%) had gall bladder in situ after ERCP. Bile duct stone recurrence was compared whether JPDD positive or gallbladder in situ. No matter they have gallbladder in situ or not, recurrence rates were higher in JPDD positive group although statistical significance was shown only in cholecystectomy group (Table 5).
The second segment of duodenum is the common site for diverticular disease, with most lesions classified as juxtapapillary duodenal diverticula (JPDD) (3-5). Currently, duodenal diverticula are considered that increase in prevalence with age and are rare prior to the third decade of life. This relationship with aging suggests that some form of degenerative process involving the local supporting structures result in the development of JPDD. In the present study, the relative prevalence of JPDD was significantly higher in elderly group and patients with JPDD were, on average, 7 yr older than comparable JPDD-free individuals. These results strongly support the proposed relationship between JPDD and aging.
Clinically, JPDD have been known associated with biliary disease, as they are believed to contribute to sphincter of Oddi dysfunction, bile flow disturbance and subsequent increase the risk of ascending bacterial infections from the duodenum (13-16). The relationship between bacteria and calcium bilirubinate pigment stones now has been widely accepted that these bacteria play an important role in lithogenesis (17, 18). Van Basten and Stockbrugger (15) reported the presence of concomitant bile duct stones in 53% of patients with JPDD and 22% of patients without JPDD. In the present study, the prevalence of JPDD was also significantly higher among patients with biliary stone disease (42.4%) when compared with patients with other comorbid biliary diseases (16.3%) or healthy controls (18.8%). As such, these results strongly suggest a causal relationship between JPDD with biliary stone formation.
Although JPDD can be diagnosed via forward-view endoscopy, a proper examination of the true lesion morphology requires side-view duodenoscopy. While several previous studies have reported JPDD prevalence ranging from 5% to 27% (with an average of 17%) (19), most of these only included patients with biliary disease. Moreover, no published data exist regarding the incidence of JPDD in healthy people, as determined by side-view duodenoscopy. In this study, the prevalence of JPDD in both biliary stone disease patients and healthy controls were pretty higher than that of other published data. It seems to be the result of close endoscopic observation to find even small diverticula. To our knowledge, our study represents the largest study of JPDD incidence among biliary stone disease patients and healthy controls by side-view duodenoscopy that has been completed to date.
Data from Van Basten and Stockbrugger (15) indicated that bile duct dilatation is significantly more common in patients with JPDD when compared with those without JPDD. However, although CBD diameters were somewhat more dilated among JPDD positive patients in our patient population, no significant differences were identified between groups. Among the JPDD subtype which was classified by proximity to the papilla, type I JPDD, in which the major papilla is located within the diverticulum, carrying a theoretically greater risk of biliary stone formation and has been known as an independent risk factor of stone recurrence (20). However, in this study, the distribution of JPDD subtype between subject groups was not statistically different, although type 1 JPDD was relatively more common (14.4%) among biliary stone disease patients when compared with patients with other co-morbid biliary diseases (3.6%). These results suggest even type 3 JPDD can be risk factor of biliary stone formation. However, it is not clear how the type 3 JPDD, especially in smaller one, could affect the bile stone formation. With regard to the complication in this study, only cannulation failure rate was higher in type 1 JPDD than type 2 and 3. However, other complications such as post-ERCP pancreatitis and stone recurrence rate were not different according to the JPDD subtype.
JPDD has been known as an independent risk factor for acute idiopathic pancreatitis and post-ERCP pancreatitis (19, 21, 22). Here, although JPDD positive patients had a significantly higher incidence of post-ERCP pancreatitis, it is unclear whether these cases of post-ERCP pancreatitis are directly related to the presence of JPDD itself rather than an associated technical difficulty (e.g., a higher rate of cannulation failure). Although several prior reports were unable to demonstrate any difference in cannulation failure rate (23), our data indicate that JPDD positive patients have significantly higher rates of cannulation failure.
In several earlier studies, JPDD was identified an independent risk factor for bile duct stone recurrence (24), and it was predominant in type 1 JPDD (20). Results from the present study also suggest that JPDD has a contributory effect on biliary stone disease recurrence, as recurrence rates were significantly higher in patients with JPDD (22.5%) when compared to JPDD-free patients (11.3%). However, in this study, significant difference was not found according to the types of JPDD. With regard to cholecystectomy, recurrence rates were higher in both group of gallbladder in situ and cholecystectomy when they have JPDD and it was statistically significant in cholecystectomy group. This result strongly supports the role of JPDD as a risk factor of biliary stone disease recurrence. However, 2 yr of observation in this study could be short to evaluate recurrence and have some limitations to rule out residual stone. Furthermore, the influence of stone removal techniques - such as sphincterotomy or papillary balloon dilatation - on the rates of recurrence was not evaluated in the present study because of two techniques were applied together not infrequently.
In conclusion, the frequency of JPDD was significantly higher in patients with biliary stone disease than in patients with other comorbid biliary diseases or healthy age-matched controls. Among patients with biliary st one disease, a direct correlation was observed between JPDD frequency and aging. Such results strongly indicate that JPDD develops with aging and likely predisposes patients to biliary stone formation. JPDD also may substantially increase the risk of post-ERCP pancreatitis, cannulation failure and biliary stone disease recurrence. Accordingly, short term follow up for recurrence and prophylactic management of complication should be considered in patients with concomitant JPDD and biliary stone disease.
Figures and Tables
Fig. 1
Juxtapapillary duodenal diverticula (JPDD) subtype classified by proximity to the major duodenal papilla. (A) Type 1 JPDD with the major duodenal papilla located within the diverticulum. (B) Type 2 JPDD with the major duodenal papilla located at the diverticular margin. (C) Type 3 JPDD with the major duodenal papilla adjacent to the diverticular margin.
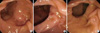
Table 1
Juxtapapillary duodenal diverticula (JPDD) frequency among individuals with biliary stone disease (group I), non-biliary stone disease (group II), and healthy controls (group III)

References
1. Zoepf T, Zoepf DS, Arnold JC, Benz C, Riemann JF. The relationship between juxtapapillary duodenal diverticula and disorders of the biliopancreatic system: analysis of 350 patients. Gastrointest Endosc. 2001. 54:56–61.
2. Lobo DN, Balfour TW, Iftikhar SY, Rowlands BJ. Periampullary diverticula and pancreaticobiliary disease. Br J Surg. 1999. 86:588–597.
3. Lane JE, Ajjan M, Sedghi S. GI bleeding from duodenal diverticula. Am J Gastroenterol. 2001. 96:2799–2800.
4. Pimparkar BD. Bokus HL, Berd JE, editors. Diverticulosis of the small intestine. Gastroenterology. 1976. 3rd ed. Philadelphia: WB Saunders;437–458.
5. Cheshire NJ, Glezer G. Zinner MJ, Schwartz SI, Ellis H, editors. Diverticula, volvulus, superior mesenteric artery syndrome and foreign bodies. Maingoats abdominal operation. 10th ed. London: Prentice Hall International (UK) Limited;916–921.
6. Harford WV. Feldman M, Scharschmidt BF, Sleisenger MH, editors. Diverticula of the hypopharynx and esophagus, the stomach and small bowel. Sleisenger and Fordtran's gastrointestinal and liver diseases. 1998. 6th ed. Philadelphia: WB Saunders;313–316.
7. Sugiyama M, Atomi Y. Periampullary diverticula cause pancreatobiliary reflux. Scand J Gastroenterol. 2001. 36:994–997.
8. Katsinelos P, Dimiropoulos S, Pilpilidis I, Galanis I, Tsolkas P, Papagiannis A, Paroutoglou G, Giouleme O, Kamperis E, Vasiliadis I, et al. Endoscopic sphincterotomy in patients with "acalculus" cholangitis associated with juxtapapillary diverticula. Hepatogastroenterology. 2004. 51:649–651.
9. van Nieuwkoop C, Boere I, Rosekrans PA, Bac DJ. Recurrent bacterial cholangitis due to a juxtapapillary diverticulum. Eur J Gastroenterol Hepatol. 2002. 14:189–190.
10. Yoneyama F, Miyata K, Ohta H, Takeuchi E, Yamada T, Kobayashi Y. Excision of a juxtapapillary duodenal diverticulum causing biliary obstruction: report of three cases. J Hepatobiliary Pancreat Surg. 2004. 11:69–72.
11. Boix J, Lorenzo-Zúñiga V, Añaños F, Domènech E, Morillas RM, Gassull MA. Impact of periampullary duodenal diverticula at endoscopic retrograde cholangiopancreatography: a proposed classification of periampullary duodenal diverticula. Surg Laparosc Endosc Percutan Tech. 2006. 16:208–211.
12. Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991. 37:383–393.
13. Shocket E, Simon SA. Small bowel obstruction due to enterolith (bezoar) formed in a duodenal diverticulum: a case report and review of the literature. Am J Gastroenterol. 1982. 77:621–624.
14. Kim MH, Myung SJ, Seo DW, Lee SK, Kim YS, Lee MH, Yoo BM, Min MI. Association of periampullary diverticula with primary choledocholithiasis but not with secondary choledocholithiasis. Endoscopy. 1998. 30:601–604.
15. van Basten JP, Stockbrügger R. Relationship between duodenal diverticuli, gallstones and duodenal and pancreaticobiliary disorders. Ned Tijdschr Geneeskd. 1996. 140:1122–1125.
16. van der Spuy S. The relationship between juxtapapillary diverticula and biliary calculi. An endoscopic study. Endoscopy. 1979. 11:197–202.
17. Miyazaki S, Sakamoto T, Miyata M, Yamasaki Y, Yamasaki H, Kuwata K. Function of the sphincter of Oddi in patients with juxtapapillary duodenal diverticula: evaluation by intraoperative biliary manometry under a duodenal pressure load. World J Surg. 1995. 19:307–312.
18. Skar V, Skar AG, Osnes M. The duodenal bacterial flora in the region of papilla of Vater in patients with and without duodenal diverticula. Scand J Gastroenterol. 1989. 24:649–656.
19. Leivonen MK, Halttunen JA, Kivilaakso EO. Duodenal diverticulum at endoscopic retrograde cholangiopancreatography, analysis of 123 patients. Hepatogastroenterology. 1996. 43:961–966.
20. Kim DI, Kim MH, Lee SK, Seo DW, Choi WB, Lee SS, Park HJ, Joo YH, Yoo KS, Kim HJ, et al. Risk factors for recurrence of primary bile duct stones after endoscopic biliary sphincterotomy. Gastrointest Endosc. 2001. 54:42–48.
21. Psathakis D, Utschakowski A, Müller G, Broll R, Bruch HP. Clinical significance of duodenal diverticula. J Am Coll Surg. 1994. 178:257–260.
22. Uomo G, Manes G, Ragozzino A, Cavallera A, Rabitti PG. Periampullary extraluminal duodenal diverticula and acute pancreatitis: an underestimated etiological association. Am J Gastroenterol. 1996. 91:1186–1188.
23. Chang-Chien CS. Do juxtapapillary diverticula of the duodenum interfere with cannulation at endoscopic retrograde cholangiopancreatography? A prospective study. Gastrointest Endosc. 1987. 33:298–300.
24. Pereira-Lima JC, Jakobs R, Winter UH, Benz C, Martin WR, Adamek HE, Riemann JF. Long-term results (7 to 10 years) of endoscopic papillotomy for choledocholithiasis. Multivariate analysis of prognostic factors for the recurrence of biliary symptoms. Gastrointest Endosc. 1998. 48:457–464.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download