Abstract
Osteoporosis is a widely recognized health problem in postmenopausal women. Osteoporotic fractures reduce independency, limit daily living activities, and increase the mortality rate. Epidemiological studies have demonstrated that low handgrip strength is a risk factor for functional limitations and disabilities, and all-cause mortality. We investigated the relationship between handgrip strength and bone mineral density (BMD) of the spine, femur neck, and total hip, as well the relationship between handgrip strength and previous fragility fractures in 337 healthy postmenopausal Korean women (mean age of 59.5 ± 6.8 yr) who were free of diseases or medications affecting bone metabolism. Age and handgrip strength were associated with BMD of the spine, femur neck, and total hip in multiple regression models. Low handgrip strength (odds ratio [OR], 0.925; range, 0.877 to 0.975; P = 0.004) and low femur neck BMD (OR, 0.019; range, 0.001 to 0.354; P = 0.008) were independent predictors of previous fragility fractures in a multiple regression model. Our results demonstrate that low handgrip strength is associated with low BMD of the spine, femur neck, and total hip, and with increased risk of previous fragility fractures.
Osteoporosis is a widely recognized health problem in postmenopausal women that is associated with increased mortality rates. Osteoporotic fractures reduce independency and limit walking performance and daily living activities, thus seriously affecting the quality of life in later years.
The handgrip dynamometer is a simple, easy, and noninvasive tool that measures muscle strength. Handgrip strength is an excellent outcome predictor of functionality, nutritional status, and mortality in elderly people (1). There are several studies in the literature with conflicting results regarding the relationship between handgrip strength and bone mineral density (BMD) at distal sites, such as the spine and femoral neck, in postmenopausal women. Some reports demonstrated a positive correlation between handgrip strength and the femoral neck BMD (2, 3), while other studies did not show a positive relationship in postmenopausal women (4-6). Two reports demonstrated that low handgrip strength was a risk factor for developing fractures, including incident vertebral fractures (2) and all fragility fractures (7).
We investigated the relationship between handgrip strength and BMD of the spine, femur neck, and total hip, as well as the association between handgrip strength and previous fragility fractures in postmenopausal Korean women.
This study was performed from June 2010 to October 2011 in Kangwon National University Hospital and involved 337 healthy postmenopausal women volunteers who satisfied the inclusion criteria. Postmenopausal women over 50 yr of were recruited; menopause was defined as the absence of menstruation for at least 1 yr. Excluded from this study were women taking glucocorticoids and estrogen for more than the preceding 3 months or women with diseases that could affect bone metabolism, such as Graves' disease or Cushing syndrome. Hysterectomized women and premenopausal bilaterally ovariectomized women were also excluded from this study.
A self-administered questionnaire, interviews, and anthropometric measurements were used to collect data on lifestyle, physical activity, and previous medical and fracture histories.
Areal BMD (g/cm2) values were determined in the spine, femoral neck, and total hip in all subjects using a Lunar Prodigy Vision dual-emission X-ray absorptiometry (DXA) system (Lunar Corp., Madison, WI, USA). According to the World Health Organization definition, osteoporosis was determined as a T-score ≤-2.5 standard deviations (SD) at any site. One investigator performed all densitometry tests and analyses in all subjects.
Maximum handgrip strength of the dominant hand was measured using the ks-301 electronic hand-held dynamometer (Lavisen, Hanam, Korea). Each subject held the dynamometer in her dominant hand with the elbow flexed and forearm parallel to the floor and squeezed the apparatus as hard as possible. The best result of two attempts was recorded in kilograms.
Daily physical activity (walking or cycling) was measured using the responses to the questionnaire ("How much time do you usually spend walking or on a bicycle outdoors each day?") Possible response included none, less than 30 min, 30 min to 1 hr, or more than 1 hr.
Pearson correlation coefficients were calculated to analyze the relationship between handgrip strength and BMD. Student's t-tests were used to compare baseline characteristics between women with and without histories of fragility fractures. Linear regression was used to investigate the association between handgrip strength and BMD, adjusting for age, body mass index (BMI), years after menopause, smoking, and physical activity. A multiple logistic regression analysis with the stepwise forward method was used to evaluate independent variables. The history of fragility fractures was set as the dependent variable. P values of < 0.05 were considered significant.
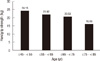
This study included 337 postmenopausal women with a mean age of 59.5 ± 6.8 yr. The subjects' mean BMI was 34.6 ± 11.3 kg/m2, mean age at menopause was 50.2 ± 4.0 yr old, and there were 67 (19.9%) women with previous fragility fractures (Table 1). Age was a strong influencing factor on handgrip strength (Fig. 1) and handgrip strength decreased with age (P < 0.001, Fig. 1). Pearson correlations showed that age, menopausal duration, and handgrip strength were associated with BMD of the spine, femur neck, and total hip region.
In a multiple linear regression model, age and handgrip strength were associated with BMD of the spine, femur neck, and total hip (Table 2). However, age, handgrip strength, smoking status, and BMI were associated with BMD of the total hip. Maximal handgrip strength was weaker in women with a history of fragility fractures compared to women without a fracture history (19.8 vs 22.6 kg, P < 0.001).
There was an association between age, postmenopausal duration, maximal handgrip strength, spine BMD, femur neck BMD, total hip BMD, and the risk of previous fragility total fractures.
A multivariate logistic regression analysis with the stepwise forward method was performed using age, menopausal duration, handgrip strength, and femur neck BMD as independent variables, and previous fragility fractures as the dependent variable. Low handgrip strength (odds ratio [OR], 0.925; range, 0.877 to 0.975; P = 0.004) and low femur neck BMD (OR, 0.019; range, 0.001 to 0.354; P = 0.008) were independent predictors of previous fragility fractures. However, handgrip strength was not an independent risk factor for previous spine fragility fractures in the multiple logistic regression models.
In this study on postmenopausal healthy women, we demonstrated that lower handgrip strength of the dominant hand is associated with reduced BMD in the spine, femoral neck, and total hip. The association could not be explained by differences in BMI, levels of activity, age, smoking status, or postmenopausal duration.
Conflicting results regarding the relationship between handgrip strength and BMD have been previously reported (2-6). Our study of postmenopausal women with a relatively large sample size (n = 337) is consistent with the majority of previous studies and reinforces the association between handgrip strength and BMD in the spine and hip.
Two prospective studies concerning the association between low handgrip strength and fractures (2, 7) have been published to date. In a study population of 1,380 women, low handgrip strength was shown to be associated with an increased risk of incident vertebral fractures, but not with prevalent fractures (2). In another study that evaluated 649 healthy postmenopausal women, handgrip strength was found to be a risk factor of incident fragility fractures (7).
We showed that low handgrip strength and low BMD are associated with increased risk of previous fragility fractures. Because the numbers of previous vertebral fractures (n = 8, 2.4%) were too small to reach statistical significance, this study failed to show an association between handgrip strength and fragility vertebral fractures.
Handgrip strength measurements are a non-invasive, low-cost, easy method for characterizing overall muscle strength. Handgrip strength is correlated with muscle function of the lower extremities (8) and lower mobility (9) in elderly adults. Handgrip strength may show general frailty (10), nutritional status (1, 11), physical activity (12), functional disability (13), dependency in activities of daily living (ADL) and cognitive decline (14), and all-cause mortality (15). Epidemiological studies demonstrated that low handgrip strength in healthy adults is a risk factor for functional limitations and disability in older age as well as for all-cause mortality (13, 16). Whereas dominant handgrip strength was shown to be associated with BMD in Korean perimenopausal and postmenopausal women (17), there were no previous studies on handgrip strength and fragility fractures in Korean women. Our multiple regression analysis showed that low handgrip strength and low femur neck BMD are risk factors of previous fragility fractures, suggesting DXA measurement could be coupled with simple handgrip strength measurements to predict fractures in postmenopausal women. This study suggests that strategies for improving muscle strength may provide protection against future risk of low BMD and may prevent fragility fractures in postmenopausal women.
This study has several limitations to be considered when interpreting the results. First, this study is a cross-sectional study with community dwelling volunteer participants, therefore our findings may not apply to the general population of postmenopausal women. Further, we did not verify the standardized radiographs to define fractures, relying instead on the interview history of fragility fractures.
In conclusion, low handgrip strength is positively associated with low BMD of the spine, femur neck, and total hip, as well as with increased risk of previous fragility fractures in postmenopausal Korean women.
Figures and Tables
References
1. Norman K, Stobäus N, Gonzalez MC, Schulzke JD, Pirlich M. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr. 2011. 30:135–142.
2. Dixon WG, Lunt M, Pye SR, Reeve J, Felsenberg D, Silman AJ, O'Neill TW. European Prospective Osteoporosis Study Group. Low grip strength is associated with bone mineral density and vertebral fracture in women. Rheumatology (Oxford). 2005. 44:642–646.
3. Kröger H, Tuppurainen M, Honkanen R, Alhava E, Saarikoski S. Bone mineral density and risk factors for osteoporosis: a population-based study of 1600 perimenopausal women. Calcif Tissue Int. 1994. 55:1–7.
4. Lindsey C, Brownbill RA, Bohannon RA, Ilich JZ. Association of physical performance measures with bone mineral density in postmenopausal women. Arch Phys Med Rehabil. 2005. 86:1102–1107.
5. Foley KT, Owings TM, Pavol MJ, Grabiner MD. Maximum grip strength is not related to bone mineral density of the proximal femur in older adults. Calcif Tissue Int. 1999. 64:291–294.
6. Bayramoğlu M, Sözay S, Karata M, Kilinç S. Relationships between muscle strength and bone mineral density of three body regions in sedentary postmenopausal women. Rheumatol Int. 2005. 25:513–517.
7. Albrand G, Munoz F, Sornay-Rendu E, DuBoeuf F, Delmas PD. Independent predictors of all osteoporosis-related fractures in healthy postmenopausal women: the OFELY study. Bone. 2003. 32:78–85.
8. Garcia PA, Dias JM, Dias RC, Santos P, Zampa CC. A study on the relationship between muscle function, functional mobility and level of physical activity in community-dwelling elderly. Rev Bras Fisioter. 2011. 15:15–22.
9. Choquette S, Bouchard DR, Doyon CY, Sénéchal M, Brochu M, Dionne IJ. Relative strength as a determinant of mobility in elders 67-84 years of age. A nuage study: nutrition as a determinant of successful aging. J Nutr Health Aging. 2010. 14:190–195.
10. Theou O, Jones GR, Jakobi JM, Mitnitski A, Vandervoort AA. A comparison of the relationship of 14 performance-based measures with frailty in older women. Appl Physiol Nutr Metab. 2011. 36:928–938.
11. Martin S, Neale G, Elia M. Factors affecting maximal momentary grip strength. Hum Nutr Clin Nutr. 1985. 39:137–147.
12. Young DR, Masaki KH, Curb JD. Associations of physical activity with performance-based and self-reported physical functioning in older men: the Honolulu Heart Program. J Am Geriatr Soc. 1995. 43:845–854.
13. Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999. 281:558–560.
14. Taekema DG, Gussekloo J, Maier AB, Westendorp RG, de Craen AJ. Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Ageing. 2010. 39:331–337.
15. Ling CH, Taekema D, de Craen AJ, Gussekloo J, Westendorp RG, Maier AB. Handgrip strength and mortality in the oldest old population: the Leiden 85-plus study. CMAJ. 2010. 182:429–435.
16. Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, Guralnik JM. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci. 2000. 55:M168–M173.
17. Ock SM, Choi WS, Song CH. The relationship between grip strength and femoral and vertebral bone mineral density in peri- and postmenopausal women. J Korean Acad Fam Med. 1999. 20:377–385.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download