Abstract
Pulmonary artery hypertension (PAH) causes right ventricular failure and possibly even death by a progressive increase in pulmonary vascular resistance. Bone marrow-derived mesenchymal stem cell therapy has provided an alternative treatment for ailments of various organs by promoting cell regeneration at the site of pathology. The purpose of this study was to investigate changes of pulmonary haemodynamics, pathology and expressions of various genes, including ET (endothelin)-1, ET receptor A (ERA), endothelial nitric oxide synthase (NOS) 3, matrix metalloproteinase (MMP) 2, tissue inhibitor of matrix metalloproteinase (TIMP), interleukin (IL)-6 and tumor necrosis factor (TNF)-α in monocrotaline (MCT)-induced PAH rat models after bone marrow cell (BMC) transfusion. The rats were grouped as the control (C) group, monocrotaline (M) group, and BMC transfusion (B) group. M and B groups received subcutaneous (sc) injection of MCT (60 mg/kg). BMCs were transfused by intravenous injection at the tail 1 week after MCT injection in B group. Results showed that the average RV pressure significantly decreased in the B group compared with the M group. RV weight and the ratio of RH/LH+septum significantly decreased in the B group compared to the M group. Gene expressions of ET-1, ERA, NOS 3, MMP 2, TIMP, IL-6, and TNF-α significantly decreased in week 4 in the B group compared with the M group. In conclusion, BMC transfusion appears to improve survival rate, RVH, and mean RV pressure, and decreases gene expressions of ET-1, ERA, NOS 3, MMP 2, TIMP, IL-6, and TNF-α.
Pulmonary arterial hypertension (PAH) is difficult to treat and is characterized by increased pulmonary arterial pressure, right heart dysfunction, lung vascular remodeling, and death (1). PAH has been shown to be refractory to most of the conventional pharmacological therapies. Although a number of therapies, including endothelin (ET)-1 antagonists, phosphodiesterase inhibitors, and prostanoid have proven useful in decreasing pulmonary arterial pressure, the long-term outcome of this condition is still bleak with a low probability of improving exercise tolerance and quality of life (2, 3).
Gene therapy has been demonstrated to have a beneficial effect in experimental models of PAH (4-6). In addition, cell-based therapies with pulmonary arterial smooth muscle cells and endothelial progenitor cells with or without transfection of therapeutic genes have a beneficial effect in experimental models of PAH (7-11).
Mesenchymal stem cells (MSCs), also known as marrow stromal cells, are a type of adult stem cells derived from the bone marrow (12). Stem cell based therapy has received attention as a possible alternative to organ transplantation, owing to the ability of stem cells to repopulate and differentiate at the engrafted site. Stem cell therapy may constitute a new treatment modality for patients with PAH (4, 13).
However, literature is rare on changes of gene expression after MSCs in the treatment of PAH. Although previous studies show that MSCs are capable of differentiating into pulmonary lineage cells in an injured lung, there is little information on changes of gene expressions after administration of MSCs in monocrotaline (MCT)-treated rats (14-16).
MCT has been reported to damage the endothelium of pulmonary arteries and induce progressive pulmonary hypertension in rats after a single subcutaneous injection (17). Also, it has been reported to impair endothelium-dependent responses in the isolated lung arteries and rat lung (18).
The purpose of this study was to investigate changes of pulmonary hemodynamics, pathology and expressions of genes, including endothelin (ET)-1, ET receptor A (ERA), endothelial nitric oxide synthase (NOS) 3, matrix metalloproteinase (MMP) 2, tissue inhibitor of matrix metalloproteinase (TIMP), interleukin (IL)-6 and tumor necrosis factor (TNF)-α in MCT-induced PAH rat model after bone marrow cell (BMC) transfusion.
Six-week-old male Sprague-Dawley rats, weighing approximately 250-300 g, were used for this study. All rats were housed in climate-controlled conditions with 12 hr light exposure: a 12 hr dark cycle, and had free access to chow food and water.
Pulmonary hypertension was induced by subcutaneous (sc) injection of 60 mg/kg MCT (Sigma Chemicals, St. Louis, MO, USA) dissolved in 0.5 N HCl solution. The rats were grouped as follows: control group (n = 12), which received sc injection of saline; M group (n = 12), which received sc injection of MCT; BMC transfusion (B) group (n = 12), in which BMC (2×107 cells) were transfused by intravenous injection at the tail 1 week after MCT injection. The rats were sacrificed in weeks 2 and 4. Lung tissues were removed and immediately frozen at -70℃ for enzyme analysis, post-fixed in 10% formalin, and processed routinely for paraffin embedding. All protocols were approved by the Institutional Animal Care and Use Committees of the School of Medicine of Ewha Womans University (approval No. ESM 11-0169).
The rats were weighed and observed for general appearance during the study period. The animals were sacrificed at the scheduled time, and the hearts and lungs were rapidly removed. The wet weights of the right ventricle (RV), left ventricle and septum (LV+S) were measured and the ratio of organ weight to body weight was calculated. The RV to LV+S ratio, (RV/[LV+S]) was used as an index of RVH.
The animals were placed in the supine position and instrumented with an arterial pressure line (Physiological Pressure Transducer, MLT1199; AD Instruments, Oxfordshire, UK). Hemodynamic parameters were recorded at baseline and in weeks 2 and 4. The catheter was placed in the RV to measure mean RVP.
Lung tissue was fixed with 10% buffered formalin with gentle perfusion through the trachea for 24 hr and then embedded in paraffin. Three µm-thick sections were stained with Victoria blue to evaluate histopathologic changes of pulmonary blood vessels. More than 20 images of pulmonary arterioles (50-160 µm diameters) per tissue section at a magnification of 400 × were captured using a microscopic digital camera and analyzed using an image analysis program (analySIS, Olympus Soft Imaging Solutions, Singapore). The external diameter (D), across the smallest diameter, and the medial wall thickness between the internal and external elastic lamina of two sides of muscular arteries (M1 and M2) were measured. The percentage of wall thickness was calculated as follows: % wall thickness = (M1 + M2)/D × 100 (Fig. 1). In addition, the number of muscular arteries accompanied by respiratory bronchioli and present in alveolar wall were counted. A total of 20 randomly selected microscopic fields per tissue section at a magnification (200 ×) were assessed.
Total RNA was extracted by using TRIzol Reagent™ (Invitrogen, Carlsbad, CA, USA), according to the Trizol method protocol and resuspended in diethyl pyrocarbonate water. The final RNA amount was spectrophotometrically determined at 260/280 nm. Quality was assessed as the absence of smear of 18S and 28S bands analyzed by means of Bio analyzer 2100 (Agilent technologies, CA, USA). RNA samples were stored at -70℃ prior to usage. cDNAs were synthesized by 1 µg of total RNA, according to the manufacture's protocol (Hight Capacity RNA-to-cDNA kit, Appllied Biosystems, CA, USA).
Real-time quantitative PCR was performed in triplicate in 384-well plates. A 384-well high-throughput analysis was performed by using the ABI Prism 7900 Sequence Detection System Software (Applied Biosystems) and white colored 384-well plates (ABgene, Hamburg, Germany) for intensification of the fluorescent signals by a factor of three. The system operated using a thermal cycler and a laser that is directed via fiber optics to each of the 384 sample wells. The fluorescence emission from each sample was collected by a charge-coupled device-camera and the quantitative data were analyzed using the Sequence Detection System Software (SDS version 2.0, Applied Biosystems). Reaction mixtures contained 10 pM/µL of each primer and 2X SYBR Green PCR Master Mix (Applied Biosystems), which includes the HotStarTaqt DNA-Polymerase in an optimized buffer, the dNTP mix (with dUTP additive), the SYBRs Green I fluorescent dye, and ROX dye as a passive reference. Each of the 384-well real-time quantitative PCR plates included serial dilutions (1, 1/2, and 1/4) of cDNA, which were used to generate relative standard curves for genes.
The resulting first-strand of cDNA were normalized by the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene. The normalized cDNA was used for the PCR procedure as a template. The specific primers for rat ET-1 were 5'-TCTCGGAGAGCAGAGACACA-3' (forward) and 5'-TGGACTTTGGAGTTTCTCCCT-3' (reverse);
The specific primers for ERA were 5'-CACAGGCTTCAGTGTGCATT-3' (forward) and 5'-CAACACAGGCCCTTAGCTTC-3' (reverse);
The specific primers for NOS 3 were 5'-CTGCGGTGATGTCACTATGG-3' (forward) and 5'-AAATGTCCTCGTGGTAGGGT-3' (reverse);
The specific primers for MMP 2 were 5'-AAGAGGCCTGGTTACCCTGT-3' (forward) and 5'-AAGTAGCACCTGGGAGGGAT-3' (reverse);
The specific primers for TIMP were 5'-GACCTATAGTGCTGGCTGTG-3' (forward) and 5'-GATCGCTCTGGTAGCCCTTCT-3' (reverse).
The specific primers for IL-6 were 5'-CCG GAG AGG AGA CTT CAC AG-3' (forward) and 5'-GATCGCTCTGGTAGCCCTTCT-3' (reverse);
The specific primers for TNF-α were 5'-GAC AGT TGA TTT CTG GGC CCT TT-3' (forward) and 5'-CCA CTG TTC TGT GCT CC-3' (reverse) (Fig. 2).
All primers were amplified using the same conditions. Thermal cycling conditions were 50℃ for 2 min and 95℃ for 10 min followed by 40 cycles of 95℃ for 30 sec and 60℃ for 30 sec, 72℃ for 30 sec. In order to exclude the presence of unspecific products, a melting curve analysis of products was performed routinely after finishing amplification by a high-resolution data collection during an incremental temperature increase from 60℃ to 95℃ with a ramp rate of 0.21℃/sec. We then converted real-time PCR cycle numbers to gene amounts (ng) on the basis of the equation. The real-time PCR analysis was performed on an Applied Biosystems Prism 7900 Sequence Detection System (PE Applied Biosystems).
The tissue was homogenized in 10 mM Tris HCl buffer, pH 7.4 containing 0.5 mM EDTA, pH 8.0, 0.25 M sucrose, 1 mM PMSF, 1 mM Na4VO3 and a protease inhibitor cocktail (Roche-Boehringer-Mannheim, IN, USA). After centrifugation, the supernatant was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Samples equivalent to 25 µg of protein content were loaded and size-separated in 10% SDS-PAGE. The proteins on the acrylamide gel were transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA) at 400 mA in a transfer buffer containing 25 mM Tris and 192 mM glycine, pH 8.4. The PVD membranes was blocked in TBS with 5% non-fat dry milk at room temperature for 1 hr in 0.1% TBS-Tween 20 and incubated with the specific primary antibodies, including ET-1, ERA, NOS 3, MMP 2, TIMP, IL-6, and TNF-α (Cell Signaling Technology Inc., Danvers, MA, USA) and GAPDH (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), at 4℃ overnight. The membrane was incubated with horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology Inc., Danvers, MA, USA) for 1 hr at room temperature. After washing, the membrane was visualized by a chemiluminescence reaction using an ECL-detection kit system (Amersham Pharmacia Biotechnology, CA, USA). The cell lysate from the solubilized tissue was mixed with the Pierce BCA reagents (Pierce, Rockford, IL, USA) and incubated for 30 min. The protein content was quantified with a Molecular Devices ELISA reader (Amersham Pharmacia Biotechnology, Sunnyvale, CA, USA) at 562 nm based on the bovine serum albumin standard curve.
To isolate donor BMCs, Sprague-Dawley rats were sacrified by cervical dislocation, and their limbs were subsequently removed. Bone marrow was flushed from the medullary cavities of both the femurs and tibias with RPMI-1640 medium (Gibco BRL, Carlsbad, CA, USA) using a 25-gauge needle. For BM-MSC isolation, C3H/he BMC were plated at a density of 2 × 107/mL/cm2, and cultured in complete MesenCult Basal Medium for Murine Mesenchymal Stem Cells (Stem Cell Technologies, Vancouver, Canada) containing mesenchymal stem cell stimulatory supplements, 100 U/mL penicillin and 100 µg/mL streptomycin (Invitrogen Life Technologies) at 37℃ in a 5% CO2 atmosphere. At 48 hr, the non-adherent cells were removed by washing with 1 × PBS, and fresh medium was then added. The medium was changed weekly. When the culture was near confluence, the monolayer cells were washed twice with 1 × PBS, and were incubated for 2-3 min at 37℃ with a 0.25% trypsin solution containing 0.01% EDTA (Invitrogen Life Technologies). Trypsin was neutralized by the addition of fresh complete medium. The resulting suspension was then expanded by plating it onto a new culture flask.
Results were expressed as the mean ± standard deviation. An unpaired two-tailed t-test and Mann-Whitney test were used to compare the three groups and a P value < 0.05 was considered statistically significant. SPSS 12.0 for Windows (SPSS, Chicago, IL, USA) was used for all statistical analyses.
There were no deaths in the control group. Four-week survival was 67% in the MCT group; and the survival was significantly increased to 87.5% in the B group (P < 0.05).
Total weight of rats significantly decreased in the M group compared with the C group in week 4. The RV weight significantly increased in the M group in weeks 2 and 4. The RV weight significantly decreased in the B group compared with the M group in weeks 2 and 4. Also, LV and septum (S) weight decreased in the M group in week 4, but no significant decrease was observed in the B group. In week 4, the RV/LV+S weight significantly decreased in the B group compared with the M group (Table 1).
The mean right ventricular pressure (RVP) significantly decreased in the B group compared with the M group in week 2 (34.8 ± 7.4 mmHg vs 15.4 ± 3.8 mmHg, P < 0.05) and in week 4 (42.3 ± 13.0 mmHg vs 14.8 ± 3.6 mmHg, P < 0.05) (Table 2).
The predominant changes in pulmonary vasculature included the development of medial wall thickening in the pulmonary arterioles in the M group compared with the C group in weeks 2 and 4. In contrast, medial wall thickness of the pulmonary arterioles appeared to be thin in the B group in week 4 (Fig. 3).
The ratio of medial thickness to the external diameter of the pulmonary artery in the M group significantly increased compared with C group in week 2 (38.8 ± 1.9% vs 17.5 ± 6.6%, P < 0.05) and in week 4 (40.2 ± 5.4% vs 19.9 ± 8.6%, P < 0.05). In the B group, the ratio of medial thickening of the pulmonary artery was significantly decreased compared with the M group in week 2 (29.3 ± 5.2% vs 38.8 ± 1.9%, P < 0.05) (Table 3).
The number of muscular pulmonary arterioles increased in the M group compared with the C group in week 2 (1.5 ± 0.1 vs 0.8 ± 0.2, P < 0.05) and in week 4 (2.0 ± 0.1 vs 0.9 ± 0.2, P < 0.05). The number of muscular pulmonary arterioles significantly decreased in the B group compared with the M group in week 4 (1.4 ± 0.3 vs 2.0 ± 0.1, P < 0.05) (Table 4).
Gene expressions of ET-1 in the M group were higher than in the C group in weeks 2 and 4. Gene expressions of ET-1 in the B group were lower than in the M group in week 4 (Fig. 4A).
The ERA gene expressions significantly increased in the M group more than in the C group in weeks 2 and 4. However, gene expressions of ERA significantly decreased in the B group than in the M group in week 4 (Fig. 4B).
Gene expressions of NOS 3 significantly increased in the M group than in the C group in weeks 2 and 4. The gene expressions of NOS 3 significantly decreased in the B group than in the M group in week 4 (Fig. 4C).
Gene expressions of MMP 2 significantly increased in the M group than in the C group in weeks 2 and 4. In week 4, the gene expressions of MMP 2 significantly decreased in the B group compared to the M group (Fig. 4D).
The gene expressions of TIMP significantly increased in the M group than in the C group in weeks 2 and 4. The gene expressions of TIMP significantly decreased in the B group than in the M group in week 4 (Fig. 5A).
The gene expressions of IL-6 significantly increased in the M group than in the C group in weeks 2 and 4. The gene expressions of IL-6 significantly decreased in the B group than the M group in week 4 (Fig. 5B).
The gene expressions of TNF-α significantly increased in the M group than in the C group in weeks 2 and 4. The gene expressions of TNF-α significantly decreased in the B group compared with the M group in week 4 (Fig. 5C).
Monocrotaline (MCT), a pyrrolizidine alkaloid derived from Crotalaria spectabilis, causes a pulmonary vascular syndrome in rats characterized by proliferative pulmonary vasculitis, PAH and cor pulmonale. In rats with MCT-induced PAH, the elevation of pulmonary arterial pressure correlates with a thickening of the medial wall in small pulmonary arteries and arterioles due to the proliferation of vascular smooth muscle cells (17, 19, 20). We have demonstrated pathologic finding and changes of gene expressions in MCT model of PAH in our previous studies (17, 19, 20) and confirmed in the present study. The evidence includes the following data: 1) RV and the ratio of RV to LV+S progressively increased in weeks 2 and 4; 2) the right ventricular pressure (RVP) progressively increased in the M group compared with the C group in weeks 2 and 4; 3) medial thickening in the pulmonary arterioles and an increased number of intra-acinar muscular arteries were noted in the M group in weeks 2 and 4; 4) gene expressions of ET-1, ERA, NOS 3, MMP 2, TIMP, IL-6, and TNF-α significantly increased in the M group in weeks 2 and 4. The pathologic findings were consistent with other results which showed a significant rise of pulmonary blood pressure and apparent RVH after MCT injection (21, 22).
Utilizing this well established model of PAH, the present study showed that an intravenous administration of BMC attenuated the MCT-induced PAH in the rat model. BMC treatment attenuated average RVH in weeks 2 and 4 and RV weight in weeks 2 and 4 in the BM group compared to the M group. RH/LH+S ratio significantly lowered in weeks 2 and 4. After BMC administration, medial wall thickness of the pulmonary arteries significantly decreased in week 2 and the number of muscular pulmonary arterioles significantly decreased in week 4 compared with the M group. Expressions of various genes, including ET1, ERA, NOS 3, MMP 2, TIMP, IL-6, and TNF-α significantly decreased in the B group compared to the M group in week 4. Changes of several gene expressions are more sensitive than those of pulmonary pathology (19). Our data is consistent with reports that BMC transplantation reduces the development of PAH by increasing vascular beds in pulmonary circulation (23). Although previous studies showed that MSCs are capable of differentiating into pulmonary lineage cells in the injured lung, there was little information about the effect of administered MSCs on pulmonary vascular resistance and endothelium-dependent responses in MCT-treated rats (14-16). Therefore, our data provide a strong rationale that stem cell therapy may constitute a new treatment modality for patients with PAH (13).
There are several possible mechanisms contributing to the efficacy of our therapeutic approach (23). MSCs are reported to have the tendency to home in on the damaged locations (24). MSCs may filter through the lungs and engraft at the sites of lung parenchymal or vascular damage. MSCs have been reported to lodge in the pulmonary circulation in PAH models by intravenous administration (25). In addition, MSCs are known to rapidly multiply and survive even under low-serum conditions (26). Thus, some have suggested that MSCs have the pluripotent ability to become endothelial progenitor cells and other cell lineages (27). Therefore, MSCs could home in to the lesion and regenerate to increase the microvascular beds, eventually leading to a decrease in vascular resistance.
Transplanted MSCs may repair injured vascular endothelium by an action involving the release of factors that improve endothelial function or stimulate vascular growth in the MCT-injured lung (11, 12, 28). Significantly decreased ET-1, ERA, and eNOS gene expressions suggest that BMC improved endothelial cell function.
The release of growth factors or cytokines has been described when stem cells are transplanted into the microenvironment of injured tissue and serve as biological catalysts for tissue repair and regeneration (28). In our study, IL-6 and TNF-α gene expressions significantly decreased in 4 week.
Baber et al. (4) published that the intratracheal injection of 3 × 106 MSCs two weeks after administration of MCT attenuated the rise in pulmonary arterial pressure and pulmonary vascular resistance when responses were evaluated in week 5. These data suggested that the decrease in pulmonary vascular resistance and improvement in response to acetylcholine an endothelium-dependent vasodilator in MCT treated rats may result from a paracrine effect of the transplanted MSCs in lung parenchyma, which improves vascular endothelial function in the MCT-injured lung.
In our study, gene expressions of ET-1, ERA, NOS 3, MMP 2, TIMP, IL-6, and TNF-α had significantly increased in the M group in weeks 2 and 4 significantly decreased in week 4 in the B group compared with the M group. These may have played a major role in the observed efficacy for PAH.
The optimal dose and frequency of BMC transfusion is not determined yet. Various timing and doses of BMC transfusion were employed by many authors (4, 13, 23). An overdose of MSCs may also lead to pulmonary embolism and rapid mortality (23). Therefore, the amount of administered MSCs is a critical parameter. To address this problem, MSCs overexpressing eNOS may be preferable over MSCs alone in the treatment of RV impairment caused by PAH because eNOS can reduce the amount of administered cells required (23). Kanki-Horimoto et al. (23) evaluated RV hypertrophy and the elevation of RV systolic pressure three weeks after MCT administration. Intravenous implantation of MSCs/eNOS ameliorated PAH-related RV impairment and survival time. The gene-transduced MSCs not only regenerated endothelial cells thereby increasing the vascular beds of pulmonary arteries but enhanced the secretion of NO from the endothelial cells. These processes are thought to decrease the constriction of pulmonary arteries and their resistance, resulting in a reduction in PAH (23).
Different modes of administration of stem cells, including intravenous (23), intratracheal (4) and direct implantation of cells into the lungs (11) have been investigated. In our study, 2 × 107 BMC were transfused by intravenous injection one week after MCT injection. The rats were sacrificed in weeks 2 and 4 to assess the response of BMC transfusion. In our pilot study, intratracheal administration of BMC led to hypoxia. We believe that the intravenous route of administration is a safe and effective method of cell injection (13). Survival rate was much improved after BMC transfusion.
Umar et al. (13) reported that rats were treated with 106 MSCs intravenously on day 14 and MSCs were obtained from donor rats with PAH 28 days after MCT. On day 28, the RV function of recipient rats was assessed, followed by isolation of the lungs and heart. The application of MSCs from donor rats with PAH reduced RV pressure overload, RV dysfunction and lung pathology in recipient rats with PAH. These results suggest that autologous MSC therapy may alleviate cardiac and pulmonary symptoms in PAH patients (13).
The limitations of our study are as follows. First, no histochemical analysis was performed in this study. Hence, whether regenerative responses including recruitment and homing of implanted MSCs occurred in the pulmonary microvasculature is unclear. Second, whether several factors other than pulmonary arterial pressure and RV hypertrophy contribute to the prolongation of survival time has not been established. Third, sample size is small and the follow-up duration is relatively short. Future studies with larger sample size and a longer follow-up duration will be required to determine the appropriate amount of BMC count, frequency and time interval required for PAH treatment.
In conclusion, BMC transfusion improves survival rate, RVH, and mean RVP, and decreases gene expressions of ET-1, ERA, NOS 3, MMP 2, TIMP, IL-6 and TNF-α in the MCT-induced PAH rat model.
Figures and Tables
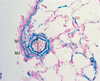 | Fig. 1Morphometric analysis of pulmonary arteries. The external diameter (D), across the smallest diameter, and the medial wall thickness between the internal and external elastic lamina of two sides of muscular arteries (M1 and M2) were measured. The percentage of wall thickness was calculated as follows: % wall thickness = (M1 + M2)/D × 100 (H&E staining, × 400). |
 | Fig. 2The RT-PCR products from the transcripts of IL-6, TNF-α, ET-1, ERA, NOS3, MMP2, TIMP and TIMP were 132 bp, 113 bp, 156 bp, 118 bp, 140 bp, 137 bp, and 133 bp respectively. TNF, tumor necrosis factor; ET-1, endothelin-1; ERA, endothelin receptor A; NOS-3, endothelial nitric oxide synthase; MMP 2, matrix metalloproteinase 2; TIMP, tissue inhibitor of matrix metalloproteinases; IL, interleukin. |
 | Fig. 3Photographs of peripheral pulmonary arteries in three groups (Victoria blue staining × 400). The medial layer of the pulmonary arterioles was progressively thickened after monocrotaline injection. The medial wall thickness of the M injection was significantly attenuated in B group. C, control; M, monocrotaline; B, bone marrow cell. |
 | Fig. 4Gene expressions of ET-1. (A) ERA, (B) NOS3, (C) MMP2, (D) after bone marrow cell transfusion. *P < 0.05 vs the corresponding value in the C group; †P < 0.05 vs the corresponding value in the M group. C, control; M, monocrotaline; B, bone marrow cell; ET-1, endothelin-1; ERA, endothelin receptor A; NOS3, nitric oxide synthase-3; MMP2, matrix metallo proteinase2. |
 | Fig. 5Gene expressions of TIMP. (A) IL-6, (B) TNF-α, (C) after bone marrow cell transfusion. *P < 0.05 vs the corresponding value in the C group; †P < 0.05 vs the corresponding value in the M group. C, control; M, monocrotaline; B, bone marrow cell; TIMP, tissue inhibitor of matrix metalloproteinases; IL-6, Interleukin-6; TNF, tumor necrosis factor. |
Table 1
Changes in body weight, right ventricle, left ventricle, RV/LV+septum and lung in each group

References
1. Gabbay E, Fraser J, McNeil K. Review of bosentan in the management of pulmonary arterial hypertension. Vasc Health Risk Manag. 2007. 3:887–900.
2. Dandel M, Kemper D, Weng Y, Hummel M, Mulahasanovic S, Kapell S, Lehmkuhl H, Hetzer R. Primary pulmonary hypertension: survival benefits of therapy with prostacyclin analogs and transplantation. Transplant Proc. 2003. 35:2117–2120.
3. McLaughlin VV, Genthner DE, Panella MM, Rich S. Reduction in pulmonary vascular resistance with long-term epoprostenol (prostacyclin) therapy in primary pulmonary hypertension. N Engl J Med. 1998. 338:273–277.
4. Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, Hyman AL, Kadowitz PJ. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007. 292:H1120–H1128.
5. Champion HC, Bivalacqua TJ, Greenberg SS, Giles TD, Hyman AL, Kadowitz PJ. Adenoviral gene transfer of endothelial nitric-oxide synthase (eNOS) partially restores normal pulmonary arterial pressure in eNOS-deficient mice. Proc Natl Acad Sci U S A. 2002. 99:13248–13253.
6. Nagaya N, Yokoyama C, Kyotani S, Shimonishi M, Morishita R, Uematsu M, Nishikimi T, Nakanishi N, Ogihara T, Yamagishi M, et al. Gene transfer of human prostacyclin synthase ameliorates monocrotaline-induced pulmonary hypertension in rats. Circulation. 2000. 102:2005–2010.
7. Campbell AI, Kuliszewski MA, Stewart DJ. Cell-based gene transfer to the pulmonary vasculature. Endothelial nitric oxide synthase overexpression inhibits monocrotaline-induced pulmonary hypertension. Am J Respir Cell Mol Biol. 1999. 21:567–575.
8. Campbell AI, Zhao Y, Sandhu R, Stewart DJ. Cell-based gene transfer of vascular endothelial growth factor attenuates monocrotaline induced pulmonary hypertension. Circulation. 2001. 104:2242–2248.
9. Nagaya N, Kangawa K, Kanda M, Uematsu M, Horio T, Fukuyama N, Hino J, Harada-Shiba M, Okumura H, Tabata Y, et al. Hybrid cell-gene therapy for pulmonary hypertension based on phagocytosing action of endothelial progenitor cells. Circulation. 2003. 108:889–895.
10. Nagaya N, Kanagawa K. Adrenomedullin in the treatment of pulmonary hypertension. Peptides. 2004. 25:2013–2018.
11. Takahashi M, Nakamura T, Toba T, Kajiwara N, Kato H, Shimizu Y. Transplantation of endothelial progenitor cells into the lung to alleviate pulmonary hypertension in dogs. Tissue Eng. 2004. 10:771–779.
12. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997. 276:71–74.
13. Umar S, de Visser YP, Steendijk P, Schutte CI, Laghmani el H, Wagenaar GT, Bax WH, Mantikou E, Pijnappels DA, Atsma DE, et al. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009. 297:H1606–H1616.
14. Grove JE, Lutzko C, Priller J, Henegariu O, Theise ND, Kohn DB, Krause DS. Marrow-derived cells as vehicles for delivery of gene therapy to pulmonary epithelium. Am J Respir Cell Mol Biol. 2002. 27:645–651.
15. Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001. 128:5181–5188.
16. Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001. 105:369–377.
17. Lim KA, Shin JY, Cho SH, Kim KW, Han JJ, Hong YM. Effect of endothelin receptor blokade on monocrotaline-induced pulmonary hypertension in rats. Korean J Pediatr. 2009. 52:689–695.
18. Ito KM, Sato M, Ushijima K, Nakai M, Ito K. Alterations of endothelium and smooth muscle function in monocrotaline-induced pulmonary hypertensive arteries. Am J Physiol Heart Circ Physiol. 2000. 279:H1786–H1795.
19. Lim KA, Kim KC, Cho MS, Lee BE, Kim HS, Hong YM. Gene expression of endothelin-1 and endothelin receptor A on monocrotaline-induced pulmonary hypertension in rats after bosentan treatment. Korean Circ J. 2010. 40:459–464.
20. Koo HS, Kim KC, Hong YM. Gene expression of nitric oxide synthase and matrix metalloproteinase-2 in monocrotaline-induced pulmonary hypertension in rats after bosentan treatment. Korean Circ J. 2011. 41:83–90.
21. Miyauchi T, Yorikane R, Sakai S, Sakurai T, Okada M, Nishikibe M, Yano M, Yamaguchi I, Sugishita Y, Goto K. Contribution of endogenous endothelin-1 to the progression of cardiopulmonary alterations in rats with monocrotaline-induced pulmonary hypertension. Circ Res. 1993. 73:887–897.
22. Itoh T, Nagaya N, Fujii T, Iwase T, Nakanishi N, Hamada K, Kanagawa K, Kimura H. A combination of oral sildenafil and beraprost ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med. 2004. 169:34–38.
23. Kanki-Horimoto S, Horimoto H, Mieno S, Kishida K, Watanabe F, Furuya E, Katsumata T. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006. 114:I181–I185.
24. Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003. 100:8407–8411.
25. Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001. 169:12–20.
26. Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, Yamagishi M, Mori H, Kangawa K, Kitamura S. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocar dial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004. 287:H2670–H2676.
27. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002. 418:41–49.
28. Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004. 94:678–685.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download