Abstract
Sauchinone has been known to have anti-inflammatory and antioxidant effects. We determined whether sauchinone is beneficial in regional myocardial ischemia/reperfusion (I/R) injury. Rats were subjected to 20 min occlusion of the left anterior descending coronary artery, followed by 2 hr reperfusion. Sauchinone (10 mg/kg) was administered intraperitoneally 30 min before the onset of ischemia. The infarct size was measured 2 hr after resuming the perfusion. The expression of cell death kinases (p38 and JNK) and reperfusion injury salvage kinases (phosphatidylinositol-3-OH kinases-Akt, extra-cellular signal-regulated kinases [ERK1/2])/glycogen synthase kinase (GSK)-3β was determined 5 min after resuming the perfusion. Sauchinone significantly reduced the infarct size (29.0% ± 5.3% in the sauchinone group vs 44.4% ± 6.1% in the control, P < 0.05). Accordingly, the phosphorylation of JNK and p38 was significantly attenuated, while that of ERK1/2, Akt and GSK-3β was not affected. It is suggested that sauchinone protects against regional myocardial I/R injury through inhibition of phosphorylation of p38 and JNK death signaling pathways.
A myocardial ischemia/reperfusion (I/R) injury is a complex process involving the generation and release of reactive oxygen species and inflammatory cytokines (1). An urgent restoration of coronary blood flow to the ischemic area is the most effective strategy to improve outcomes of myocardial I/R injury. Upon resuming the perfusion, reperfusion injury salvage kinases (RISK) survival signaling pathways such as phosphatidylinositol-3-OH kinases (PI3K)-Akt, extra-cellular signal-regulated kinases (ERK1/2), and their downstream target glycogen synthase kinase (GSK)-3β may be activated to confer tissue protection (2). However, at the same time, the reperfusion may also activate 'stress-responsive' mitogen-activated protein kinase (MAPK) subfamilies (socalled cell death signaling pathway), i.e., c-Jun N-terminal kinases (JNK) and p38 MAPKs, resulting in necrosis (3). Therefore, the degree of I/R injury may be determined by a relative extent of death and survival kinases activation (4).
Sauchinone is a biologically active lignan isolated from Saururus chinensis which has been long used in folk medicine for the treatment of edema, jaundice, and several inflammatory diseases (5). It has been known to inhibit apoptotic death of C6 glioma cells induced by staurosporine (6) and to reduce ischemia-induced neuronal death via suppression of intracellular radical production (7). We have previously shown that sauchinone inhibits lipopolysaccaride-induced tumor necrosis factor-α (TNF-α) expression in macrophages via suppression of NF-κB activation (8).
The present study aimed at examining whether sauchinone would provide a protection against regional myocardial I/R injury. The expression of molecules related with cell survival such as Akt and ERK1/2/GSK-3β as well as that related with cell death such as JNK and p38 MAPKs was determined.
The procedures were carried out after the approval by the institutional animal care and use committee, Research Institute of Medical Science at Chonnam National University Medical School (Gwangju, Korea). Male Sprague-Dawley rats weighing 250-350 g were used. They were housed in a vivarium maintained at 20℃-23℃ with 12-hr light/dark cycle and given food and water ad libitum. On the experimental day, the rats were anesthetized with phenobarbital (100 mg/kg, intraperitoneal), and mechanically ventilated with an air-oxygen mixture. The polyethylene catheter was cannulated into the common carotid artery for the arterial blood pressure monitoring and into the internal jugular vein for drug administration (9). Core body temperature was monitored with a rectal temperature probe and maintained at 37.5℃-38.5℃ using heating pad. Left thoracotomy was performed at the fifth intercostal space, and 6-0 silk ligature was placed around left anterior descending coronary artery (LAD). After stabilization period of 20 min, the LAD was occluded for 20 min and then reperfused for 2 hr. LAD occlusion was confirmed by ST segment changes in electrocardiogram and the presence of regional cyanosis in the myocardial surface. There were three I/R groups as shown in Fig. 1: 1) control group, 2) sauchinone group which received sauchinone (10 mg/kg, dissolved in dimethyl subfoxide [DMSO]), and 3) DMSO group which received vehicle only. Sauchinone was isolated from the n-hexane fraction of Saururus chinensis by successive silica gel chromatography and reverse-phase high-pressure liquid chromatography, and was > 97% pure (10). The dosage of sauchinone adopted was effective to increase survival rates and to decrease the plasma level of TNF-α in mice administered lipopolysaccharide/D-galactosamine (10). Sauchinone and DMSO were administered intraperitoneally 30 min before the onset of ischemia. At the end of 2 hr reperfusion, the heart was quickly excised and mounted on a Langendorff apparatus. The LAD was reoccluded, and then fluorescent polymer microspheres were infused into the aorta to demarcate the risk zone as the tissue without fluorescence. The heart was sliced into 2 mm transverse sections, which were incubated at 37℃ for 20 min in triphenyl tetrazolium chloride in sodium phosphate buffer. The slices were immersed in 10% formalin to enhance the contrast between stained (viable) and unstained (necrotic) tissue and then squeezed between glass plates spaced exactly 2 mm apart. The myocardium at risk was identified by illuminating the slices with ultraviolet light. The infracted and risk zone regions were traced on a clear acetate sheet and quantified with Image Tool (San Antonio, TX, USA). The areas were converted into volumes by multiplying the areas by slice thickness. Infarct size is expressed as a percentage of the risk zone (11).
For immunoblotting, the heart was excised 5 min after reperfusion, and the risk area in the left ventricle was separated in each group. Myocardial samples were homogenized and equivalent amounts of proteins were loaded to 10% Tris-HCl SDS polyacrylamide gel. Proteins were electrotransferred to a polyvinylidene difluoride membrane. The membrane was then incubated overnight at 4℃ with rat polyclonal specific primary antibodies to phosphorylated p38, JNK, Akt, ERK1/2 and GSK-3β (Ser9) (Cell Signaling Technology; Danvers, MA, USA). It was subsequently incubated with anti-rabbit or anti-rat immunoglobulin HRP-coupled secondary antibodies. Immunoreactive bands were visualized using ECL Western blotting detection reagents. The membranes were then stripped using a stripping buffer, and reprobed with antibodies specific for total p38, JNK, Akt, ERK1/2, GSK-3β and β-actin (Cell Signaling Technology). Data were expressed as means ± SD. Differences in infarct size and immunoblot data between groups were tested by one-way analysis of variance (ANOVA). A Scheffé test was used for multiple pair-wise comparisons when a significant difference was indicated with ANOVA. P < 0.05 was considered to be statistically significant.
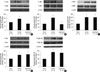
The heart rate and arterial pressure did not significantly differ among the groups at any time point (data not shown). Nor did the ratio of the area at risk to left ventricular mass differ among the groups. Sauchinone significantly reduced the infarct size (Fig. 2). Sauchinone significantly attenuated the degree of phosphorylation of p38 and JNK (P < 0.05), without affecting that of ERK1/2, Akt and GSK-3β (Fig. 3).
Our study demonstrated that sauchinone has a novel protective effect against regional myocardial I/R injury in an open-chest rodent model. The protective effect was associated with attenuated phosphorylation of cell death signaling pathways (p38 and JNK), without effects on that of RISK pathways (ERK1/2, Akt) and GSK-3β. Several recent studies have suggested that activation of p38 MAPK and JNK, known enhancers of the expression of adhesion molecules and the production of cytokines (12), plays a major role in cell death following I/R injury. The concept has been supported by the observations in which an inhibition of p38 activation reduced the caspase-3 activity and protected cardiac myocytes following I/R insult in vitro (13), and improved postischemic cardiac function in vivo (14). It was also shown that an inhibition of JNK decreased ischemia-induced necrosis and apoptosis in myocardial I/R injury (15, 16). However, an activation of p38 MAPK or JNK has been also shown to play a protective role in I/R injury (17, 18). The distinction of beneficial versus detrimental role of p38 and/or JNK signaling remains to be further addressed.
On the other hand, the extent of phosphorylation of Akt, ERK and GSK-3β induced by I/R was not affected by sauchinone in the present study, suggesting that RISK/GSK-3β pathways were not involved. The disturbance of ion homeostasis resulting from ATP depletion following the opening of the mitochondrial permeability transition pore (mPTP) can lead to necrotic and apoptotic cell death (19). Both ischemic/pharmacological pre- and post-conditioning are generally perceived to protect the heart against I/R injury via activation of the RISK pathway involving PI3K-Akt and ERK1/2 at the time of reperfusion (2). Its activation may prevent cellular damages by converging on the mPTP through GSK-3β, which in turn inhibits its opening (19). Sauchinone (EC50 = 1 µM) has been shown to prevent mitochondrial dysfunction and apoptosis induced by iron plus arachidonic acid in the hepatocyte, possibly through inhibition of reactive oxygen species production (20). Further studies defining the role of mPTP in sauchinone-induced myocardial protection against I/R injury may be needed.
In summary, the present study demonstrates a cardioprotective effect of sauchinone against regional I/R injury in the rat, which may be related to an attenuation of cell death signaling pathways involving p38 and JNK but not to activation of RISK/GSK-3β pathways.
Figures and Tables
Fig. 1
Experimental protocol. Sauchinone (10 mg/kg) and dimethyl sulfoxide (DMSO) were administered intraperitoneally 30 min before ischemia.

Fig. 2
Effects of sauchinone on the infarct size. All rats were subjected to 20 min regional ischemia followed by 2 hr of reperfusion. The infarct size is expressed as a percentage of area at risk. Sauchinone reduced infarct size, while DMSO alone was without effects. Values are mean ± SD. *P < 0.05 vs control.

Fig. 3
Effects of sauchinone on the expression of total and phosphorylated p38 (A), JNK (B), ERK1/2 (C), Akt (D), and GSK-3β (E). Sauchinone (10 mg/kg) attenuated the phosphorylation of p38 and JNK, while it did not affect that of ERK1/2, Akt and GSK-3β. The expression of total p38, JNK, ERK1/2, Akt, or GSK-3β was not altered by sauchinone. Upper panels show representative immunoblots, and lower panels show densitometric data. Sham group was without I/R. Values are mean ± SD. *P < 0.05 vs sham; †P < 0.05 vs control.
References
1. Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. 2004. 61:481–497.
2. Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004. 61:448–460.
3. Yin T, Sandhu G, Wolfgang CD, Burrier A, Webb RL, Rigel DF, Hai T, Whelan J. Tissue-specific pattern of stress kinase activation in ischemic/reperfused heart and kidney. J Biol Chem. 1997. 272:19943–19950.
4. Yue TL, Wang C, Gu JL, Ma XL, Kumar S, Lee JC, Feuerstein GZ, Thomas H, Maleeff B, Ohlstein EH. Inhibition of extracellular signal-regulated kinase enhances ischemia/Reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res. 2000. 86:692–699.
5. Sung SH, Kim YC. Hepatoprotective diastereomeric lignans from Saururus chinensis herbs. J Nat Prod. 2000. 63:1019–1021.
6. Song H, Kim YC, Moon A. Sauchinone, a lignan from Saururus chinensis, inhibits staurosporine-induced apoptosis in C6 rat glioma cells. Biol Pharm Bull. 2003. 26:1428–1430.
7. Choi IY, Yan H, Park YK, Kim WK. Sauchinone reduces oxygen-glucose deprivation-evoked neuronal cell death via suppression of intracellular radical production. Arch Pharm Res. 2009. 32:1599–1606.
8. Bae HB, Li M, Son JK, Seo CS, Chung SH, Kim SJ, Jeong CW, Lee HG, Kim W, Park HC, et al. Sauchinone, a lignan from Saururus chinensis, reduces tumor necrosis factor-alpha production through the inhibition of c-raf/MEK1/2/ERK 1/2 pathway activation. Int Immunopharmacol. 2010. 10:1022–1028.
9. Kim SJ, Yoo KY, Jeong CW, Kim WM, Lee HK, Bae HB, Kwak SH, Li M, Lee J. Urinary trypsin inhibitors afford cardioprotective effects through activation of PI3K-Akt and ERK signal transduction and inhibition of p38 MAPK and JNK. Cardiology. 2009. 114:264–270.
10. Seo CS, Lee YK, Kim YJ, Jung JS, Jahng Y, Chang HW, Song DK, Son JK. Protective effect of lignans against sepsis from the roots of Saururus chinensis. Biol Pharm Bull. 2008. 31:523–526.
11. Song DK, Jang Y, Kim JH, Chun KJ, Lee D, Xu Z. Polyphenol (-)-epigallocatechin gallate during ischemia limits infarct size via mitochondrial K(ATP) channel activation in isolated rat hearts. J Korean Med Sci. 2010. 25:380–386.
12. Srivastava S, Weitzmann MN, Cenci S, Ross FP, Adler S, Pacifici R. Estrogen decreases TNF gene expression by blocking JNK activity and the resulting production of c-Jun and JunD. J Clin Invest. 1999. 104:503–513.
13. Mackay K, Mochly-Rosen D. An inhibitor of p38 mitogen-activated protein kinase protects neonatal cardiac myocytes from ischemia. J Biol Chem. 1999. 274:6272–6279.
14. Li Z, Ma JY, Kerr I, Chakravarty S, Dugar S, Schreiner G, Protter AA. Selective inhibition of p38α MAPK improves cardiac function and reduces myocardial apoptosis in rat model of myocardial injury. Am J Physiol Heart Circ Physiol. 2006. 291:H1972–H1977.
15. Ferrandi C, Ballerio R, Gaillard P, Giachetti C, Carboni S, Vitte PA, Gotteland JP, Cirillo R. Inhibition of c-Jun N-terminal kinase decreases cardiomyocyte apoptosis and infarct size after myocardial ischemia and reperfusion in anaesthetized rats. Br J Pharmacol. 2004. 142:953–960.
16. Milano G, Morel S, Bonny C, Samaja M, von Segesser LK, Nicod P, Vassalli G. A peptide inhibitor of c-Jun NH2-terminal kinase reduces myocardial ischemia-reperfusion injury and infarct size in vivo. Am J Physiol Heart Circ Physiol. 2007. 292:H1828–H1835.
17. Weinbrenner C, Liu GS, Cohen MV, Downey JM. Phosphorylation of tyrosine 182 of p38 mitogen-activated protein kinase correlates with the protection of preconditioning in the rabbit heart. J Mol Cell Cardiol. 1997. 29:2383–2391.
18. Dougherty CJ, Kubasiak LA, Prentice H, Andreka P, Bishopric NH, Webster KA. Activation of c-Jun N-terminal kinase promotes survival of cardiac myocytes after oxidative stress. Biochem J. 2002. 362:561–571.
19. Nishihara M, Miura T, Miki T, Tanno M, Yano T, Naitoh K, Ohori K, Hotta H, Terashima Y, Shimamoto K. Modulation of the mitochondrial permeability transition pore complex in GSK-3beta-mediated myocardial protection. J Mol Cell Cardiol. 2007. 43:564–570.
20. Kim YW, Lee SM, Shin SM, Hwang SJ, Brooks JS, Kang HE, Lee MG, Kim SC, Kim SG. Efficacy of sauchinone as a novel AMPK-activating lignan for preventing iron-induced oxidative stress and liver injury. Free Radic Biol Med. 2009. 47:1082–1092.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download