Abstract
There are no accurate data on the relationship between nodal station and diagnostic performance of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). We evaluated the impact of nodal station and size on the diagnostic performance of EBUS-TBNA in patients with non-small cell lung cancer (NSCLC). Consecutive patients who underwent EBUS-TBNA of mediastinal or hilar lymph nodes for staging or diagnosis of NSCLC were included in this retrospective study. Between May 2009 and February 2010, EBUS-TBNA was performed in 373 mediastinal and hilar lymph nodes in 151 patients. The overall diagnostic sensitivity, specificity, accuracy and negative predictive value (NPV) of EBUS-TBNA were 91.6%, 98.6%, 93.8%, and 84.3%, respectively. NPV of the left side nodal group was significantly lower than those of the other groups (P = 0.047) and sensitivity of the left side nodal group tended to decrease (P = 0.096) compared with those of the other groups. Diagnostic sensitivity and NPV of 4L lymph node were 83.3% and 66.7%, respectively. However, diagnostic performances of EBUS-TBNA did not differ according to nodal size. Bronchoscopists should consider the impact of nodal stations on diagnostic performances of EBUS-TBNA.
Nodal metastasis is the most important prognostic factor in patients with non-small cell lung cancer (NSCLC) and affects therapeutic strategies (1, 2). Median survival decreases progressively as nodal metastasis increases (3). Although radiological modalities such as computed tomography (CT) scan or integrated positron emission tomography (PET)/CT scan provide significant information, radiographic staging alone does not ensure accurate nodal staging in NSCLC patients because of its relatively low sensitivity and specificity (4). Thus, all candidates for curative surgical treatment require histopathological assessment of nodal involvement (5, 6). Although mediastinoscopy has been the gold standard for nodal staging, it is an invasive technique, requires general anesthesia, has a morbidity of 2%, and has a mortality of 0.08% (5).
Recently, EBUS-TBNA was introduced as a minimally invasive technique for nodal staging and many previous studies have shown that EBUS-TBNA affords excellent diagnostic performance with a sensitivity of 69%-99.1% and NPV of 11%-98.9% (7-9). Additionally, it allows for access to the hilar and interlobar lymph nodes, which are inaccessible with mediastinoscopy (10). However, despite these advantages, some authors have indicated that EBUS-TBNA has a relatively high false negative rate compared with mediastinoscopy and have claimed that mediastinoscopy is still required as a gold standard (11, 12).
Studies have been performed on the factors associated with the diagnostic performance of EBUS-TBNA and have suggested an association between nodal station and diagnostic performance (12-14). However, there are no accurate data on the relationship between nodal station and diagnostic performance and other factors affecting diagnostic performance. Thus, the aim of this study was to evaluate the overall diagnostic performance and impact of nodal station and nodal size as influencing factors on the diagnostic performance of EBUS-TBNA for nodal staging in NSCLC patients.
In this retrospective study, records were reviewed for all patients who underwent EBUS-TBNA of mediastinal and hilar lymph nodes for diagnosis or staging of NSCLC at the Samsung Medical Center, between May 2009 and February 2010. All patients underwent a conventional diagnostic work up, consisting of a physical examination, laboratory investigations, chest X-ray, sputum cytology, and transthoracic fine-needle aspiration in cases of peripheral lung lesions. Chest CT and integrated PET/CT scans were conducted in all patients prior to EBUS-TBNA. EBUS-TBNA was performed for nodal staging in patients with pathologically confirmed NSCLC, and for diagnosis and nodal staging in patients with radiologically suspicious NSCLC.
All patients in whom nodal metastases were detected by EBUS-TBNA underwent multimodality treatment, chemotherapy, radiation or best supportive care considering disease stage, performance status, and age. If both benign and malignant results were revealed by EBUS-TBNA among the patients who underwent EBUS-TBNA for multiple lymph nodes, all negative results of lymph node(s) by EBUS-TBNA were not immediately confirmed by mediastinoscopy or lymph node dissection since treatments could be determined based on malignant results of lymph nodes. If only benign results were revealed by EBUS-TBNA among the patients who underwent EBUS-TBNA for multiple lymph nodes, negative result of mediastinal lymph nodes was confirmed by mediastinoscopy or lymph node dissection. Lymph nodes that had benign EBUS-TBNA results but that were not confirmed by surgical sampling and lymph nodes that had non-diagnostic EBUS-TBNA results were excluded from diagnostic performances analysis.
All lymph nodes that included analysis were categorized into several groups according to nodal station as proposed by the IASCL lymph node map (15), and nodal size.
After fasting for at least 6 hr before PET/CT examination, the patients received an intravenous injection of 370 MBq of 18F-FDG and then rested for 45 min before undergoing imaging. Image acquisition was performed using an integrated PET/CT device (Discovery LS, GE Healthcare, Milwaukee, WI, USA) that consisted of an Advance NXi PET scanner and an 8-slice Light Speed Plus CT scanner. Lymph nodes were classified as positive on 18F-FDG PET/CT if mediastinal and hilar lymph nodes had increased 18F-FDG uptake compared with the background activity of the surrounding mediastinal or lung tissues.
The indications for EBUS-TBNA for mediastinal and hilar lymph nodes were 1) the presence of mediastinal or hilar lymph node with a short axis diameter of ≥ 10 mm on chest CT or 2) mediastinal or hilar lymph node with increased FDG uptake compared with surrounding tissue on PET/CT scan regardless of size.
EBUS-TBNA was performed using a flexible ultrasonic puncture bronchoscope with a linear scanning transducer (BF-UC260F-OL8, Olympus, Tokyo, Japan). TBNA biopsies were performed using a dedicated 22-gauge needle (NA-201SX-4022, Olympus). All procedures were performed under conscious sedation using midazolam. Local anesthesia was achieved by nebulization with 4% lidocaine. An examination of all mediastinal and hilar node stations accessible by EBUS was performed before TBNA procedure. If more than one node was detected, EBUS-TBNA was performed at all accessible node stations. N3 nodes were sampled first, and then N2, and N1 nodes were sampled. We attempted at least three passes at each node as possible. When tissue core was obtained, we attempted at least two passes as possible (16). All aspirate specimens were expelled onto glass slides, smeared, fixed immediately, and sent for cytological and/or histological examination. Rapid on-site cytopathologic evaluation (ROSE) was not performed, and all procedures were performed by two bronchoscopists.
All aspirate samples were categorized by pathological report. The presence of frank malignant cells or rare cells suspicious for malignancy was considered malignant. The presence of no tumor cells in a background of lymphoid tissue was considered benign. Samples that showed only blood, mucus, benign bronchial epithelial cells or no lymphoid tissue were considered non-diagnostic and inadequate. All specimens were evaluated by an experienced lung pathologist.
Lymph nodes identified as malignant by EBUS-TBNA or surgical sampling were considered positive result. Lymph nodes identified as benign by EBUS-TBNA and by surgical sampling were accepted as true negatives.
All data are presented as median (range) or number (%). The sensitivity, specificity, diagnostic accuracy and negative predictive value (NPV) of EBUS-TBNA and PET/CT were calculated using the standard definitions. The overall diagnostic performance of EBUS-TBNA and the difference in diagnostic performance in relation to each nodal station and size were evaluated on a per-nodal station basis. Differences in diagnostic performance in relation to nodal station and size were evaluated with the chi-square test or Fisher's exact test; P values of < 0.05 were considered to indicate statistical significance. All statistical analyses were performed using the PASW Statistics 18 software.
EBUS-TBNA was performed in 373 mediastinal and hilar lymph nodes of 151 patients with NSCLC between May 2009 and February 2010. Characteristics of the 151 study patients are shown in Table 1. The median age of these patients was 65 yr, and 117 were men. Adenocarcinoma and squamous cell carcinoma accounted for approximately 90%. Among the 151 patients, EBUS-TBNA detected nodal metastases in 83 patients (1 was revealed as false positive). During the study period, there was no serious complication.
Fig. 1 shows the results of lymph nodes sampled by EBUS-TBNA. Of the total of 373 nodes, 143 were identified as malignant by EBUS-TBNA. Exceptionally, two (station 4R, 7) of 143 nodes subsequently underwent surgical sampling, because only small malignant foci were detected on pathological reports. One (4R) of the two nodes was revealed as malignant, but the other (station 7) was revealed as benign and accepted as a false positive result. Of the 222 nodes that were benign by EBUS-TBNA, 83 subsequently underwent surgical sampling, 70 of these 83 nodes were revealed as benign, and 13 were revealed as malignant. However, 139 nodes that had benign EBUS-TBNA results but that were not confirmed by surgical sampling and 8 nodes that had non-diagnostic EBUS-TBNA results were excluded from diagnostic performances analysis. Two non-diagnostic results from EBUS-TBNA were confirmed as benign by surgical sampling.
The characteristics of lymph nodes included in the analysis are shown in Table 2. In total, 226 nodes were included in the analysis, 196 were subcarinal and paratracheal lymph nodes. A total of 215 aspirate samples contained tissue cores. Median size of lymph nodes was 11 (4-51) mm and median number of passes per lymph node was 2 (1-5).
The overall diagnostic sensitivity, specificity, accuracy and NPV of EBUS-TBNA on a per-nodal basis were 91.6% (95% confidence interval [CI], 86.2%-95.0%), 98.6% (95% CI, 92.4%-99.8%), 93.8% (95% CI, 89.95%-96.3%) and 84.3% (95% CI, 75.0%-90.6%), respectively (Table 3). The diagnostic performances tended to decrease in the order of station 7, 4R, 4L, and 11L, especially in terms of sensitivity and NPV.
When all lymph nodes included in the analysis were categorized into a mediastinal node group and hilar/interlobar node group, there was no significant difference in diagnostic performance between the groups (Table 4). However, when categorized into three nodal groups, - midline (3P and 7), right side (1R, 2R, 4R, 10R, and 11R), and left side (4L, 10L, and 11L) -, NPV of the left side nodal group was significantly lower than those of the other groups (P = 0.047) and sensitivity of the left side nodal group tended to decrease (P = 0.096) compared with those of the other groups (Table 4).
To evaluate the effect of nodal size on diagnostic performances, all lymph nodes included in the analysis were categorized into three groups according to the short axis diameter measured by chest CT (Table 5). As the nodal size increased, the extent of metastatic involvement increased, and for the nodal size of ≥ 20 mm group, there was no false negative result. However, diagnostic performances of EBUS-TBNA did not differ according to nodal size (P = 0.0305, > 0.558).
Among 226 lymph nodes which were included in the diagnostic performance analyses, PET/CT scans were available for 225 lymph nodes. The overall diagnostic sensitivity, specificity, accuracy and NPV of PET/CT scan on a per-nodal basis were 72.9% (95% CI, 65.4%-79.3%), 77.1% (95% CI, 66.0%-85.4%), 56.3% (95% CI, 46.3%-65.7%) and 74.2% (95% CI, 68.1%-79.5%), respectively.
In this study, the overall sensitivity, specificity, accuracy and NPV of EBUS-TBNA for nodal staging in NSCLC on a per-nodal basis were 91.6%, 98.6%, 93.8%, and 84.3%, respectively, similar to those of previous studies showing excellent diagnostic performance (7-9). Diagnostic performances of EBUS-TBNA were superior to those of PET/CT scan in this study. Interestingly, we found relevance between the nodal station and diagnostic performance of EBUS-TBNA. In our study, false negative results were identified at station 7 (3 nodes), 4R (4 nodes), 4L (4 nodes), and 11L (2 nodes). When all lymph nodes were categorized into the three groups, NPV and sensitivity of the left nodal station group were lower than those of the other nodal station groups (P = 0.047 for NPV and P = 0.096 for sensitivity). Diagnostic sensitivity and NPV of 4L lymph node were 83.3% and 66.7%, respectively. Therefore, our data suggest that negative EBUS-TBNA results of left paratracheal lymph node should be confirmed by other modalities such as EUS-FNA or mediastinoscopy out of concern for low NPV.
An association between nodal station and diagnostic performance has been suggested in some previous studies. Szlubowski et al. (14) reported that imaging and biopsy of paratracheal nodes, particularly on left side, by EBUS-TBNA were technically more difficult in a study that evaluated the efficacy of EBUS-TBNA for nodal staging in 226 NSCLC patients. They reported false negative results at station 4R (3 nodes), 4L (2 nodes), and 7 (8 nodes), and compared diagnostic performances between the subcarinal node group and paratracheal node group. However, there was no difference in diagnostic performance. Additionally, Cerfolio et al. (12) reported diagnostic performances for nodal staging of NSCLC patients by EBUS-TBNA and EUS-FNA and concluded that EBUS-TBNA had high false negative rates, especially at stations 4R, 4L, and 7. Although these studies had some limitations, they suggested that nodal station may affect the diagnostic performance of EBUS-TBNA. The reason is probably related to the anatomical structure. For example, nodes at station 4L are located very close to the subaortic area and deep in the trachea. Thus, visualizing and sampling at station 4L by EBUS-TBNA is relatively more difficult than at the others (14). Because of these difficulties, several studies have been performed to identify additional yield of the combined approach of EBUS-TBNA and EUS-FNA for nodal staging in NSCLC patients and have shown additional benefits (17, 18).
Generally, lymph nodes with a short axis diameter of ≥ 10 mm on CT are considered abnormal. In our data, however, the extent of metastatic involvement in the nodal group with a short axis diameter of < 10 mm was 48.8% (42/86), which is a considerable value. Additionally, there was no statistically significant difference in diagnostic performance between the differently sized nodal groups. These data suggest that nodal size is not an important factor affecting diagnostic performance of EBUS-TBNA (19).
In our study, false negative results were seen in 13 lymph nodes from 11 patients, and the false negative rate was only 8.3% (13/155). Generally, false negative results occur at variable rates, and even in experienced hands, false negative rates of 15%-20% may be seen (20). Perhaps because we obtained tissue core samples in 95.1% and at least three aspirations as possible (at least 2 aspirations when a tissue core specimen was obtained) (16) in 81.4% (184/226) of all sampled nodes, the false negative rate in our study was low.
We experienced a patient who had discrepant results of station 7 from EBUS-TBNA (malignant) and mediastinoscopy (benign). The patient was diagnosed with squamous cell carcinoma in the left upper lobe and had high FDG uptake in the mediastinal nodes (2R, 4R, 7) on PET/CT. EBUS-TBNA was performed in the order of 2R, 4R, and 7 and reported as "suggestive of metastatic carcinoma" at station 7 on histology. Because small foci of malignancy were observed in this specimen, the patient subsequently underwent mediastinocopy, which revealed benign results at stations 7, and 4L. False positive results have been reported in other EBUS-TBNA and EUS-FNA studies (13, 21), but were uncommon, an accurate frequency and causes have not been reported. Some authors indicated that false positive results can occur if TBNA is performed through an area of bronchial epithelial high grade dysplasia or carcinoma in situ, especially at station 7 (20). Considering that all other nodes were benign, the possibility of contamination during EBUS-TBNA seems low. Other possible explanation of the positive result of station 7 from EBUS-TBNA is that mediastinoscopy result was false negative (5).
There are some limitations to our study. First, we did not perform ROSE. Some studies have suggested that ROSE may improve the diagnostic performance of TBNA in evaluating of lymphadenopathy (22, 23). However, other recent studies have shown that ROSE does not affect diagnostic performance, but only allows for avoidance of unnecessary biopsies and reduces the complication rate (24, 25). Moreover, a recent study reported some cases of discrepancy between the ROSE and final diagnosis of EBUS-TBNA (26). Thus, considering that we obtained tissue cores in 95.1%, the use of ROSE would have had little influence in our study. Second, relatively large numbers (147 nodes) were excluded from the analysis because in this retrospective study, not all patients received surgical sampling for benign lymph nodes from EBUS-TBNA. Finally, most lymph nodes with positive EBUS-TBNA results were not subsequently confirmed by surgical sampling because of known high positive predictive value of EBUS-TBNA (5, 7) and the risk of surgical sampling. Thus, an accurate false positive EBUS-TBNA result could not be evaluated.
In conclusion, bronchoscopists should consider the impact of nodal stations on diagnostic performances of EBUS-TBNA.
Figures and Tables
Fig. 1
Results of lymph nodes sampled by EBUS-TBNA. LN, lymph node; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; CCRT, concurrent chemoradiotherapy.

Table 3
Diagnostic performances of EBUS-TBNA in relation to each nodal station (%)
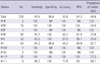
#1R, right low cervical, supraclavicular, and sternal notch nodes; #2R, right paratracheal nodes; #3P, retrotracheal nodes; #4R/4L, right/left lower paratracheal nodes; #7, subcarinal nodes; #10R/10L, right/left hilar nodes; #11R/11L, right/left interlobar nodes; NPV, negative predictive value; NA, not available.
References
1. Takamochi K, Oh S, Suzuki K. Prognostic evaluation of nodal staging based on the new IASLC lymph node map for lung cancer. Thorac Cardiovasc Surg. 2010. 58:345–349.
2. De Leyn P, Lardinois D, Van Schil PE, Rami-Porta R, Passlick B, Zielinski M, Waller DA, Lerut T, Weder W. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg. 2007. 32:1–8.
3. Robinson LA, Ruckdeschel JC, Wagner H Jr, Stevens CW. American College of Chest Physicians. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007. 132:243S–265S.
4. Silvestri GA, Gould MK, Margolis ML, Tanoue LT, McCrory D, Toloza E, Detterbeck F. American College of Chest Physicians. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest. 2007. 132:178S–201S.
5. Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA. American College of Chest Physicians. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007. 132:202S–220S.
6. Lardinois D, De Leyn P, Van Schil P, Porta RR, Waller D, Passlick B, Zielinski M, Lerut T, Weder W. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg. 2006. 30:787–792.
7. Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009. 33:1156–1164.
8. Gu P, Zhao YZ, Jiang LY, Zhang W, Xin Y, Han BH. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer. 2009. 45:1389–1396.
9. Adams K, Shah PL, Edmonds L, Lim E. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax. 2009. 64:757–762.
10. Ernst A, Eberhardt R, Krasnik M, Herth FJ. Efficacy of endobronchial ultrasound-guided transbronchial needle aspiration of hilar lymph nodes for diagnosing and staging cancer. J Thorac Oncol. 2009. 4:947–950.
11. Shrager JB. Mediastinoscopy: still the gold standard. Ann Thorac Surg. 2010. 89:S2084–S2089.
12. Cerfolio RJ, Bryant AS, Eloubeidi MA, Frederick PA, Minnich DJ, Harbour KC, Dransfield MT. The true false negative rates of esophageal and endobronchial ultrasound in the staging of mediastinal lymph nodes in patients with non-small cell lung cancer. Ann Thorac Surg. 2010. 90:427–434.
13. Szlubowski A, Herth FJ, Soja J, Kolodziej M, Figura J, Cmiel A, Obrochta A, Pankowski J. Endobronchial ultrasound-guided needle aspiration in non-small-cell lung cancer restaging verified by the transcervical bilateral extended mediastinal lymphadenectomy: a prospective study. Eur J Cardiothorac Surg. 2010. 37:1180–1184.
14. Szlubowski A, Kuzdzał J, Kołodziej M, Soja J, Pankowski J, Obrochta A, Kopiski P, Zieliński M. Endobronchial ultrasound-guided needle aspiration in the non-small cell lung cancer staging. Eur J Cardiothorac Surg. 2009. 35:332–335.
15. Giroux DJ, Rami-Porta R, Chansky K, Crowley JJ, Groome PA, Postmus PE, Rusch V, Sculier JP, Shepherd FA, Sobin L, Goldstraw P. International Association for the Study of Lung Cancer International Staging Committee. The IASLC Lung Cancer Staging Project: data elements for the prospective project. J Thorac Oncol. 2009. 4:679–683.
16. Lee HS, Lee GK, Lee HS, Kim MS, Lee JM, Kim HY, Nam BH, Zo JI, Hwangbo B. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest. 2008. 134:368–374.
17. Hwangbo B, Lee GK, Lee HS, Lim KY, Lee SH, Kim HY, Lee HS, Kim MS, Lee JM, Nam BH, Zo JI. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest. 2010. 138:795–802.
18. Khoo KL, Ho KY. Endoscopic mediastinal staging of lung cancer. Respir Med. 2011. 105:515–518.
19. Herth FJ, Ernst A, Eberhardt R, Vilmann P, Dienemann H, Krasnik M. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically normal mediastinum. Eur Respir J. 2006. 28:910–914.
20. Cameron SE, Andrade RS, Pambuccian SE. Endobronchial ultrasound-guided transbronchial needle aspiration cytology: a state of the art review. Cytopathology. 2010. 21:6–26.
21. Annema JT, Versteegh MI, Veseliç M, Welker L, Mauad T, Sont JK, Willems LN, Rabe KF. Endoscopic ultrasound added to mediastinoscopy for preoperative staging of patients with lung cancer. JAMA. 2005. 294:931–936.
22. Davenport RD. Rapid on-site evaluation of transbronchial aspirates. Chest. 1990. 98:59–61.
23. Diette GB, White P Jr, Terry P, Jenckes M, Rosenthal D, Rubin HR. Utility of on-site cytopathology assessment for bronchoscopic evaluation of lung masses and adenopathy. Chest. 2000. 117:1186–1190.
24. Trisolini R, Cancellieri A, Tinelli C, Paioli D, Scudeller L, Casadei GP, Parri SF, Livi V, Bondi A, Boaron M, Patelli M. Rapid on-site evaluation of transbronchial aspirates in the diagnosis of hilar and mediastinal adenopathy: a randomized trial. Chest. 2011. 139:395–401.
25. Baram D, Garcia RB, Richman PS. Impact of rapid on-site cytologic evaluation during transbronchial needle aspiration. Chest. 2005. 128:869–875.
26. Monaco SE, Schuchert MJ, Khalbuss WE. Diagnostic difficulties and pitfalls in rapid on-site evaluation of endobronchial ultrasound guided fine needle aspiration. Cytojournal. 2010. 7:9.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download