Abstract
Malnutrition and inflammation are related to high rates of morbidity and mortality in hemodialysis patients. Resistin is associated with nutrition and inflammation. We attempted to determine whether resistin levels may predict clinical outcomes in hemodialysis patients. We conducted a prospective evaluation of 100 outpatients on hemodialysis in a single dialysis center (male, 46%; mean age, 53.7 ± 16.4 yr). We stratified the patients into 4 groups according to quartiles of serum resistin levels. During the 18-month observational period, patients with the lowest quartile of serum resistin levels had poor hospitalization-free survival (log rank test, P = 0.016). After adjustment of all co-variables, patients with the lowest quartile of serum resistin levels had poor hospitalization-free survival, compared with reference resistin levels. Higher levels of interleukin-6 were an independent predictor of poor hospitalization-free survival. In contrast, serum resistin levels were not correlated with interleukin-6 levels. The current data showed that low resistin levels may independently predict poor hospitalization free survival in hemodialysis patients.
Morbidity and mortality in patients on hemodialysis is still high despite significant improvement in dialysis technology (1). Their hospitalization may give rise to increasing individual and socioeconomical burden. Malnutrition and inflammation are related to high rates of morbidity and mortality in patients on hemodialysis (2, 3). Serum levels of interleukin 6 (IL-6), leptin and adiponectin are known to be elevated in dialysis patients (4-6), and have been associated with morbidity and mortality of dialysis patients through an endothelial injury, pathophysiology of atherosclerotic vascular disease.
Resistin, one of the adipocytokine, is from inflammatory cells infiltrating fat tissue, not directly from adipocytes (7). It causes impairment of glucose tolerance in mice; however, this physiologic role is not related to insulin resistance in humans. Instead, it is known to have an association with inflammation, patient's nutrition, and residual renal function on hemodialysis (7, 8). However, few studies on the effect of resistin on clinical outcomes in dialytic patients have been conducted. Therefore, we attempted to determine whether resistin levels may independently predict clinical outcomes in hemodialysis patients.
We enrolled adult outpatients on hemodialysis at the Gachon University of Medical and Science Gil Hospital, Korea. Patients who had started on dialysis for less than 3 months, had a recent history of infection, malignancy, amyloidosis or structural heart disease, or cardiovascular events, or had a life-expectancy of less than 6 months for any reason were excluded from this study. A total of 100 adult patients on hemodialysis was enrolled for analysis. All the patients were dialyzed with bio-compatible membranes (F5 or F6; Fresenius Medical Care AG, Bad Homberg, Germany) using Fresenius 4008H (Fresenius Medical Care AG). Ninety seven patients underwent regular dialysis three times per week and 3 patients underwent regular dialysis two times per week. Ninety-six patients were anuric.
We prospectively observed all hospitalization events as well as demographic and laboratory data over an 18-month period. Hospitalization was defined as any hospitalization events, regardless of causes for admission, with more than one overnight stay. The primary clinical outcome was hospitalization-free survival, and the secondary clinical outcomes were annual hospitalization days and annual hospitalization frequency. Hospitalization-free survival was defined as time to first hospitalization in prospective period. Annual hospitalization days were the days of all hospital stay per year, expressed as days per patient-year. Annual hospitalization frequency was defined as number of hospital admissions per year, expressed as times per patient-year. Causes of hospitalization included 5 categories; infection-related events, dialysis-related events (hyperkalemia, vascular access related events, volume overload/dehydration), cardiovascular event, bone-related disorder (fracture and parathyroidectomy) and other events.
All of the laboratory parameters were measured in patients starting a mid-week dialytic session. Fasting blood samples were drawn from the arterial end of the vascular access just before starting hemodialysis. Serum samples were taken and centrifuged; supernatants were separated and immediately frozen at -70℃ for later analysis. Serum urea nitrogen, creatinine, hemoglobin, albumin, total cholesterol, triglyceride, and other biochemistry parameters were measured by standard laboratory techniques using an automatic analyzer (ADVIA 2004 Chemistry system [Siemens, Diagnostics, Tarrytown, IL, USA]). Single pool Kt/Vurea was calculated using the Daugirdas equation and normalized protein nitrogen appearance by Depner and Daugirdas (9, 10).
For survival analysis, patients were divided according to quartiles based on the serum levels of resistin. Resistin levels were determined using the Bio-Plex Pro human diabetes assay (Biorad Laboratories, Hercules, CA, USA). Serum IL-6 concentration was analyzed using the Bio-Plex multiplex cytokine assay (Biorad Laboratories).
Data were reported as mean ± SD or number (percentage). Parameters among the 4 separated groups were analyzed using the Kruskal-Wallis test. Survival analyses were performed using Kaplan-Meier and the Cox proportional hazard model was applied to adjust for co-variables, including age, sex, diabetes mellitus, body mass index (BMI), serum albumin, normalized protein nigtrogen appearance (nPNA), hemodialysis duration, Kt/Vurea, hemoglobin, erythropoietin resistance index, and IL-6 levels. Parameters that did not demonstrate a Gaussian distribution were logarithmically transformed. Data analysis was conducted using SPSS 11.0 for Windows (SPSS Inc., Chicago, IL, USA). A P < 0.05 was considered statistically significant.
Baseline characteristics are listed in Table 1. The mean age of patients was 53.7 ± 16.3 yr, male 46%, and the mean hemodialysis vintage was 4.3 ± 4.4 yr. Fifty three percent of patients had diabetes mellitus. Mean resistin levels were 10.0 ± 0.43 ng/mL. Patient groups were divided according to each quartile of resistin levels (Table 1). There were no significant differences in age, gender, body mass index, biochemical data, hematologic data, and dialysis parameters between the four groups. However, nPNA appeared to show a statistically significant increase according to quartile of serum resistin (P = 0.031).
There were 133 admission events and 2,096 admission days in 100 patients. Mean observational periods were 585 days. A total of 54 patients experienced at least one more admission events during the observational period. Two patients died and 1 patient underwent kidney transplantation during the observational period. Hospitalization events included 28.5% for infection-related events, 12.2% for dialysis-related events, 11.3% for vascular access related events, 8.1% for cardiovascular events, 1.6% for bone-related disorder, and 38.2% for other events (ophthalmic surgery, endoscopic procedure, and adverse effect of medication).
Hospitalization-free survival according to quartile of serum resistin is shown in Fig. 1. We defined the second quartile group as reference, the patients with the lowest quartile of serum resistin levels had poor hospitalization-free survival (P = 0.016). Low serum resistin levels, nPNA, hemodialysis vintage, diabetes mellitus, hemoglobin, and IL-6 were significant predictors for all-cause hospitalization in univariable analysis. After adjustment of all co-variables, lowest quartile of serum resistin levels, higher levels of serum IL-6, and presence of diabetes mellitus were found to independently increase the probability for hospitalization (Table 2).
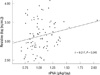
Annual hospitalization frequency and hospitalization days were 0.62 ± 1.05 times per patient-year and 8.11 ± 21.5 days per patient-year. Among these patients, frequency of annual hospitalization and hospitalization days of patients with the lowest quartile of serum resistin level were 0.81 ± 0.83 times per patient-year and 10.19 ± 19.6 days per patient-year. Annual hospitalization frequency and annual hospitalization days appeared to be high in patients with the lowest quartile of serum resistin levels; however, they were not statistically significant (Table 3). nPNA showed positive correlation with serum resistin levels (Fig. 2). Serum albumin, BMI, and IL-6 did not show significant correlation with serum resistin levels.
We demonstrated that the lowest quartile of serum resistin level predicted hospitalization-free survival, and that it was independent of other inflammatory cytokines. Although some studies have demonstrated an association of serum resistin levels with inflammation, renal function, and nutrition, respectively (8, 11-14), few studies reported on the direct relationship between resistin levels and clinical outcomes.
Resistin is comprised of 108 amino acid peptide molecules; its weight is 12.5 kDa. The kidney is the major route to elimination of resistin (15); therefore, resistin is elevated in patients with end-stage renal disease. Patients with anorexia nervosa had lower serum resistin levels (16-18), and serum resistin levels showed positive correlation with nPNA in dialytic patients (19), suggesting that there may be a significant relationship between serum resistin and nutritional status. In our data, serum resistin levels were positively correlated with nPNA. It could be an explanation that patients with low resistin levels had poor hospitalization-free survival. In survival analysis, low levels of serum resistin was a predictor of hospitalization. In addition, the group with highest quartile of serum resistin had also poor hospitalization-free survival. It suggests that there may not be linear dose-response relationship, but be U-shaped relationship between resitin levels and hospitalizaition. Kalantar-Zadeh et al. (20) indicated that low levels of serum cholesterol or low levels of body mass index in the setting of under-nutrition are short-term markers of mortality in dialysis patients. Reverse epidemiology can exist in the relationship between short term clinical outcomes and serum resistin levels as a nutritional biomarker.
Serum resistin levels were significantly correlated with value of nPNA; but the correlation coefficient was not strongly positive. It may be caused by mean value of nPNA in the group with 4th quartile which was insignificantly lower than in the group with 3rd quartile. Resistin levels are reported inversely correlated with the protein catabolic rate in a small number cross-sectional study (n = 33) (21). In contrast, resistin levels are positively correlated with body fat mass in a relatively large number cross-sectional study (n = 114) (22). Further large-scaled clinical study may be needed.
Barreto et al. (23) reported an association of interleukin-6 with mortality in predialytic and dialytic patients. In this study, higher level of IL-6 was found to significantly increase the risk for poor hospitalization-free survival. In our data, no significant difference was observed between serum resistin levels and IL-6 levels. Although resistin has a potential role in inflammation or cardiovascular disease (24, 25), most studies were cross-sectional design. Few studies on resistin observed longitudinal clinical outcomes unlike studies on leptin or adiponectin. Some reports have shown no correlation between resistin and inflammatory cytokines (26, 27). It is possible that resistin levels may predict poor hospital-free survival independently of inflammation in our hemodialysis patients.
Many studies have used hospitalization as clinical predictor for outcome (28, 29). We chose hospitalization-free survival as clinical outcomes. Unfortunately, we failed to reveal the relationship between resistin levels and death, or cardiovascular mortality, because there were only 2 death and 10 admission events for cardiovascular disease during 1-yr observation period. We did not check other nutritional parameters including prealbumin, triceps/biceps skin-fold thickness. Since we measured levels of resistin only at the baseline, we had no data on change in serum resistin levels, which may have some different effects on hospitalization. To elucidate underlying mechanism of the potential link between resistin and nutrition or inflammation, further experimental or large-scale investigation may warrant.
In conclusion, it is suggested that low resistin levels may predict poor hospitalization-free survival, independent of inflammation markers in hemodialysis patients.
Figures and Tables
ACKNOWLEDGMENTS
We thank all of the medical and nursing staff of the dialysis center at Gachon University Gil Hospital for their assistance. We also appreciate the work done by the research-nursing staff (Hee Eun Nam, Hyomi Jeong, and Ji Hye Park) with regard to collection of clinical data.
References
1. Jin DC. Current status of dialysis therapy in Korea. Korean J Intern Med. 2011. 26:123–131.
2. Carrero JJ, Stenvinkel P. Inflammation in end-stage renal disease: what have we learned in 10 years? Semin Dial. 2010. 23:498–509.
3. Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003. 42:864–881.
4. Bologa RM, Levine DM, Parker TS, Cheigh JS, Serur D, Stenzel KH, Rubin AL. Interleukin-6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients. Am J Kidney Dis. 1998. 32:107–114.
5. Merabet E, Dagogo-Jack S, Coyne DW, Klein S, Santiago JV, Hmiel SP, Landt M. Increased plasma leptin concentration in end-stage renal disease. J Clin Endocrinol Metab. 1997. 82:847–850.
6. Zoccali C, Mallamaci F, Tripepi G, Benedetto FA, Cutrupi S, Parlongo S, Malatino LS, Bonanno G, Seminara G, Rapisarda F, Fatuzzo P, Buemi M, Nicocia G, Tanaka S, Ouchi N, Kihara S, Funahashi T, Matsuzawa Y. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 2002. 13:134–141.
7. Guzik TJ, Mangalat D, Korbut R. Adipocytokines: novel link between inflammation and vascular function? J Physiol Pharmacol. 2006. 57:505–528.
8. Malyszko J, Malyszko JS, Kozminski P, Pawlak K, Mysliwiec M. Elevated resistin is related to inflammation and residual renal function in haemodialysed patients. Nephrology (Carlton). 2007. 12:246–253.
9. Daugirdas JT. Simplified equations for monitoring Kt/V, PCRn, eKt/V, and ePCRn. Adv Ren Replace Ther. 1995. 2:295–304.
10. Depner TA, Daugirdas JT. Equations for normalized protein catabolic rate based on two-point modeling of hemodialysis urea kinetics. J Am Soc Nephrol. 1996. 7:780–785.
11. Nüsken KD, Kratzsch J, Wienholz V, Stohr W, Rascher W, Dötsch J. Circulating resistin concentrations in children depend on renal function. Nephrol Dial Transplant. 2006. 21:107–112.
12. Axelsson J, Bergsten A, Qureshi AR, Heimburger O, Bárány P, Lönnqvist F, Lindholm B, Nordfors L, Alvestrand A, Stenvinkel P. Elevated resistin levels in chronic kidney disease are associated with decreased glomerular filtration rate and inflammation, but not with insulin resistance. Kidney Int. 2006. 69:596–604.
13. Liakopoulos V, Mertens PR, Eleftheriadis T, Koukoulis G, Stefanidis I. Is there a link between inflammation, plasma resistin levels, and protein malnutrition in hemodialysis patients? Kidney Int. 2006. 70:1371–1372.
14. Fagerberg B, Fagerlund C, Hulthe J. Resistin and GFR. Kidney Int. 2006. 70:1371.
15. Kielstein JT, Becker B, Graf S, Brabant G, Haller H, Fliser D. Increased resistin blood levels are not associated with insulin resistance in patients with renal disease. Am J Kidney Dis. 2003. 42:62–66.
16. Mak RH, Cheung W. Adipokines and gut hormones in end-stage renal disease. Perit Dial Int. 2007. 27:S298–S302.
17. Małgorzewicz S, Aleksandrowicz-Wrona E, Owczarzak A, Debska-Slizień A, Rutkowski B, Łysiak-Szydłowska W. Adipokines and nutritional status for patients on maintenance hemodialysis. J Ren Nutr. 2010. 20:303–308.
18. Wang QY, Zhang H, Yan X, Kang J, Yu RJ. Serum resistin and leptin in patients with chronic obstructive pulmonary disease and their relationship to nutritional state. Zhonghua Jie He He Hu Xi Za Zhi. 2005. 28:445–447.
19. Dostalova I, Kunesova M, Duskova J, Papezova H, Nedvidkova J. Adipose tissue resistin levels in patients with anorexia nervosa. Nutrition. 2006. 22:977–983.
20. Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003. 63:793–808.
21. Filippidis G, Liakopoulos V, Mertens PR, Kiropoulos T, Stakias N, Verikouki C, Patsidis E, Koukoulis G, Stefanidis I. Resistin serum levels are increased but not correlated with insulin resistance in chronic hemodialysis patients. Blood Purif. 2005. 23:421–428.
22. Yannakoulia M, Yiannakouris N, Blüher S, Matalas AL, Klimis-Zacas D, Mantzoros CS. Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resistin concentrations in healthy humans. J Clin Endocrinol Metab. 2003. 88:1730–1736.
23. Barreto DV, Barreto FC, Liabeuf S, Temmar M, Lemke HD, Tribouilloy C, Choukroun G, Vanholder R, Massy ZA. European Uremic Toxic Work Group (EUTox). Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 2010. 77:550–556.
24. Cohen G, Hörl WH. Resistin as a cardiovascular and atherosclerotic risk factor and uremic toxin. Semin Dial. 2009. 22:373–377.
25. Chang JH, Jung JY, Lee HH, Chung W, Joo KW, Kim S. Serum resistin as a novel marker of erythropoietin resistance in nondiabetic patients on hemodialysis. Tohoku J Exp Med. 2011. 224:281–285.
26. Díez JJ, Iglesias P, Fernández-Reyes MJ, Aguilera A, Bajo MA, Alvarez-Fidalgo P, Codoceo R, Selgas R. Serum concentrations of leptin, adiponectin and resistin, and their relationship with cardiovascular disease in patients with end-stage renal disease. Clin Endocrinol (Oxf). 2005. 62:242–249.
27. Taskapan MC, Taskapan H, Sahin I, Keskin L, Atmaca H, Ozyalin F. Serum leptin, resistin, and lipid levels in patients with end stage renal failure with regard to dialysis modality. Ren Fail. 2007. 29:147–154.
28. Bommer J, Locatelli F, Satayathum S, Keen ML, Goodkin DA, Saito A, Akiba T, Port FK, Young EW. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004. 44:661–671.
29. Yano A, Nakao K, Sarai A, Akagi S, Kihara T, Morimoto H, Nakamura A, Hiramatsu M, Nagake Y, Makino H. Elevated serum interleukin-18 levels might reflect the high risk of hospitalization in patients on peritoneal dialysis. Nephrology (Carlton). 2005. 10:576–582.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download