Abstract
The aim of this study was to compare the clinical outcome and cost-effectiveness of preoperative biliary drainage (BD) methods in periampullary cancer, and to suggest guidelines for selecting the appropriate preoperative BD method. Between October 2004 and August 2010, 211 patients underwent pancreatoduodenectomy after preoperative BD. Clinical outcome and cost-effectiveness of the preoperative BD methods were compared based on the final drainage method used and on intention-to-treat analysis. There was no significant difference in drainage duration between percutaneous transhepatic biliary drainage (PTBD) and endoscopic BD groups (14.2 vs 16.6 days, respectively; P = 0.121) but daily diminution of serum bilirubin level was higher in the PTBD group (0.7 vs 0.6 mg/dL/day, respectively; P = 0.041). Based on intention-to-treat analysis, drainage duration was shorter (13.2 vs 16.5 days, respectively; P = 0.049), daily diminution of serum bilirubin level was higher (0.7 vs 0.6 mg/dL/day, respectively; P = 0.041). Medical care cost was lower (14.2 vs 15.7 × 103 USD, respectively; P = 0.040) in the PTBD group than in the endoscopic BD group. When selecting the preoperative BD method, practitioners should consider that PTBD is more cost-effective and safer than endoscopic BD.
The clinical benefit of preoperative biliary drainage (BD) in periampullary cancer with obstructive jaundice is not well established (1-7). However, preoperative BD is still performed in many cases, for example, when planning preoperative chemoradiotherapy, for preoperative risk evaluation, in preexisting cholangitis or liver function abnormality, for severe malnutrition, and when expecting long waiting times for surgery due to insufficient admission beds (8, 9).
Drainage can be accomplished either externally through percutaneous transhepatic biliary drainage (PTBD) or internally through endoscopic naso-biliary drainage (ENBD) or endoscopic retrograde biliary drainage (ERBD). PTBD makes preoperative cholangiography possible and it can be left in place after an operation. It also makes postoperative decompression and cholangiography possible (10, 11). However, cholangitis, bile peritonitis due to bile leakage, bleeding, pain at insertion site, tube dysfunction, body fluid loss with electrolyte imbalance are also possible (3, 10-12). On the other hand, both diagnosis and intervention are possible when using ENBD and ERBD. These two endoscopic methods have been experimentally shown to have beneficial effects on restoring nutritional status and immune function and reducing endotoxemia (8, 15). However, reflux of duodenal contents may result in cholangitis or pancreatitis. Duodenal perforation, bleeding, and tube dysfunction may also be possible when using endoscopic BD (8-11, 13).
To our knowledge, there are few studies comparing these BD methods (14, 15). In the absence of strong evidence, selection of a preoperative BD method in a clinical situation depends on clinician's or hospital facility's preferences. The aims of this study were to compare the clinical outcome and cost-effectiveness of these preoperative BD methods in periampullary cancer, considering the final drainage method used and by using intention-to-treat analysis, and to suggest guidelines for selecting an appropriate preoperative BD method.
Clinicopathologic data were prospectively collected in electronic medical record form and retrospectively reviewed. A consecutive series of 343 patients who underwent preoperative BD at Seoul National University Hospital, for periampullary cancer with obstructive jaundice, between October 2004 and August 2010 were included. Preoperative BD was performed when the patient had cholangitis or poor liver function, or when pathologic confirmation or recovery of general condition was needed in patients with severe comorbidity. Surgery was performed after management for underlying cardiovascular or pulmonary diseases was completed and two weeks after improvement of cholangitis and restoration of liver function tests. Of the 343 patients, 55 were excluded because they underwent operations other than pancreatoduodenectomy, and 77 patients with BD previously performed at other hospitals were also excluded. As a result, 211 patients were included in this study. The preoperative BD methods were PTBD, ENBD, and ERBD. The ENBD and ERBD patients were considered as a single endoscopic BD group. Tumor locations were the ampulla of Vater, distal common bile duct (CBD), pancreas, and duodenum. Operation types were Whipple's operation and pylorus preserving pancreatoduodenectomy (PPPD). Daily diminution of serum bilirubin was calculated using the initial and final, preoperative serum bilirubin levels and the drainage duration. The definitions of all complications, as well as of their management, were evaluated according to generally accepted criteria (16-19). We estimated the patient's total hospital cost by adding up all associated costs from the day the patient visited for diagnosing and performing preoperative BD to the day the patient was discharged following performance of the pancreatoduodenectomy.
Thirty-nine patients who were initially subjected to endoscopic BD were switched to PTBD due to a procedure failure or an ineffective endoscopic BD. Therefore, clinical outcome and cost-effectiveness of each preoperative biliary drainage methods were compared on the basis of the final drainage method that was used and on results of intention-to-treat analysis.
Results are expressed as means and ranges or as the number and percentage of patients. The software program SPSS for Windows (version 19.0; SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. One-way analysis of variance was used to compare means of continuous variables. Univariate comparisons for all categorical variables were performed by using the chi-square test or Fisher's exact test, as appropriate. A probability (P) value ≤ 0.05 was considered significant.
There were 211 patients who underwent pancreatoduodenectomy at our hospital between October 2004 and August 2010. Of those, 136 patients (64.5%) were male and 75 (35.5%) were female. The mean age was 64.8 yr (range 35-89). Of the total, 191 patients (90.5%) underwent PPPD and 20 (9.5%) underwent Whipple's operation. Forty-seven patients (22.3%) had adenocarcinoma of the ampulla of Vater, 80 (37.9%) had adenocarcinoma of the distal CBD, 78 (37.0%) had adenocarcinoma of the pancreas, and 6 (2.8%) had adenocarcinoma of the duodenum. Eighty-one patients (38.4%) had cholangitis before the preoperative BD procedure. Based on the TNM staging system, 141 patients (66.8%) were T3 and 99 (46.9%) were node positive (Table 1).
Based on the patient's final BD status, 107 patients (50.7%) underwent PTBD, 53 (25.1%) underwent ERBD, and 51 (24.2%) underwent ENBD. Considering tumor location, PTBD was more frequently performed than endoscopic BD in pancreas cancer (43.9% vs 29.8%) while endoscopic BD was more frequently performed than PTBD in distal CBD or ampulla of Vater cancer (70.2% vs 50.4%) (P = 0.001). PPPD was more frequently performed than Whipple's operation in both BD groups. There were no significant differences in operation time, intraoperative blood loss, T stage, and N stage between the PTBD and endoscopic BD groups (Table 2).
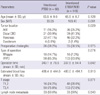
Thirty-nine patients (18.5%) who initially underwent endoscopic BD were switched to PTBD due to procedure failure (n = 37, 94.9%) or ineffective endoscopic BD (n = 2, 5.1%). On an intention-to-treat basis, 68 patients (32.2%) underwent intentional PTBD and 143 patients (67.8%) underwent intentioned endoscopic BD. Intentioned PTBD was more frequently performed than intentioned endoscopic BD in pancreas cancer (47.1% vs 32.2%) while intentioned endoscopic BD was more frequently performed than intentioned PTBD in distal CBD (30.9% vs 41.3%) or ampulla of Vater cancer (16.2% vs 25.2%) (P = 0.029). There was no significant difference in operation time, intraoperative blood loss, T stage, and N stage between the intentioned PTBD and intentioned endoscopic BD groups (Table 3).
Overall the initial serum bilirubin level was 11.2 ± 6.9 mg/dL, and the final serum bilirubin level was 3.7 ± 3.3 mg/dL, and mean BD duration was 15.4 ± 11.2 days. There was no significant difference in drainage duration between PTBD and endoscopic BD groups (14.2 vs 16.6 days, respectively; P = 0.121); however, daily diminution of serum bilirubin level was higher in the PTBD group (0.7 vs 0.6 mg/dL/day, respectively; P = 0.041) (Table 4). Within the endoscopic BD group, the ERBD subgroup had a longer drainage duration than the ENBD subgroup (19.0 vs 14.1 days, respectively; P = 0.029), but daily diminution of serum bilirubin was comparable between the two groups (0.5 vs 0.6 mg/dL/day, respectively; P = 0.339) (Table 5, Fig. 1).
On an intention-to-treat basis, drainage duration was shorter (13.2 vs 16.5 days, respectively; P = 0.049) and daily diminution of serum bilirubin level was higher (0.7 vs 0.6 mg/dL/day, respectively; P = 0.041) in the intentioned PTBD group than in the intentioned endoscopic BD group (Table 4).
Procedure-related complications are outlined in Table 6. One or more complications occurred in 62 of the patients in the study (29.4%). Among the 6 PTBD patients (5.6%) with a bleeding complication, 3 underwent gelfoam embolization without further complication. Pancreatitis occurred more frequently in the endoscopic BD group than in the PTBD group (16.3% vs 2.8%, respectively; P = 0.001). Two of the pancreatitis patients in the endoscopic BD group had to wait for surgery for two weeks in order to treat their pancreatitis before surgery. Thirty-nine patients who were initially subjected to endoscopic PBD were switched to PTBD due to procedure failure (94.9%) or ineffective BD (5.1%).
On an intention-to-treat basis, bleeding (8.8% vs 2.1%, respectively; P = 0.024) and bile peritonitis (2.9% vs 0%, respectively; P = 0.039) occurred more often in the intentioned PTBD group than in the intentioned endoscopic BD group. Pancreatitis occurred more frequently in intentioned endoscopic BD group than in the PTBD group (0% vs 14.0%, respectively; P = 0.001) (Table 6).
The overall postoperative complication rate was comparable between the PTBD and endoscopic BD groups (39.3% vs 47.1%, respectively; P = 0.155). On an intention-to-treat basis, there was no significant difference in postoperative complication rate between the intentioned PTBD group and the intentioned endoscopic BD group (Table 7).
There was no significant difference in total hospital cost, admission cost, hospital stay duration between the PTBD and endoscopic BD groups. On an intention-to-treat basis, there was no significant difference in total hospital cost and hospital stay duration, but admission cost was significantly lower in the intentioned PTBD group than in the intentioned endoscopic BD group (14.2 ± 3.9 × 103 USD vs 15.7 ± 7.0 × 103 USD, respectively; P = 0.040) (Table 8).
In 4 of 5 randomized trials, preoperative BD by means of PTBD was the standard mode of drainage used, whereas endoscopic BD is currently preferred in some hospitals, although evidence to support this preference is lacking (2, 20, 21). PTBD enables preoperative and postoperative biliary decompression and cholangiography, but complications such as cholangitis, bile peritonitis, bleeding, pain at insertion site, tube dysfunction, and electrolyte imbalance may occur. Endoscopic BD methods make both diagnosis and intervention possible, which give chance to direct visualization of duodenum and papilla, biopsy for tissue diagnosis, and direct cholangiography. However, reflux of duodenal contents may result in cholangitis, pancreatitis, duodenal perforation, or bleeding. Hwang et al. (22) reported that the thickness and degree of inflammation of the CBD wall was more severe in their endoscopic BD group, and indicated that CBD inflammation may have resulted from reflux of duodenal contents. There are few comparative studies comparing these BD methods (14, 15); therefore, in the absence of strong evidence, selection of a preoperative BD method in many cases depends on clinician's or hospital facility's preferences.
In this study, clinical outcome and cost-effectiveness were compared between three different preoperative BD methods. Considering tumor location, PTBD was more frequently performed than ENBD or ERBD in pancreas cancer, whereas the latter two were more frequently performed than PTBD in distal CBD or ampulla of Vater cancer, both on a final status basis and on an intention-to-treat basis. In distal CBD or ampulla of Vater cancer, identification of the lesion and tissue confirmation using an endoscopic procedure is possible and, on that basis, would be preferred to the use of PTBD.
We evaluated effectiveness by comparing drainage duration and daily serum bilirubin decline. On the basis of the final drainage method used, there was no difference in drainage duration between the PTBD and endoscopic BD groups, but daily diminution of the serum bilirubin level was higher in the PTBD group. The result was similar on an intention-to-treat basis, except for drainage duration: the drainage duration was shorter in the intentioned PTBD group than in the intentioned endoscopic BD group. Thirty-nine intentioned endoscopic group patients were changed to PTBD and the number of days spent between the switch is the reason for the difference in drainage duration. Additional time is needed to perform PTBD for patients with failed endoscopic attempts. In addition, time is needed to determine whether the serum bilirubin level declines and, if not, check the endoscopic BD function and the perform PTBD. Based on the difference in the decreases in bilirubin levels, the effectiveness of preoperative BD is better with PTBD than with endoscopic BD. However, the optimal duration of BD before surgery has not been established. Experimental and clinical studies have suggested that a period of at least 4-6 weeks is needed for restoration of normal major synthesis and clearance functions of the liver, as well as of mucosal intestinal barrier functions (5). In addition, depressed cell-mediated immunity, impaired hepatic reticuloendothelial function (23), and altered lymphocyte transformation have been documented, and these functions are unlikely to improve within 4 weeks (24, 25). On the other hand, increasing drainage duration increases the risks of stent clogging and secondary inflammatory changes to the bile duct wall. If such complications require readmission to a hospital, this might lead to surgery postponement (7). However, it is not easy to maintain preoperative BD over 4 weeks in a clinical setting. This study was not a randomized controlled trial, therefore the preoperative BD method used was selected according to the clinician's preference. However, the time to perform the required operation after BD was decided consistently as described earlier (i.e. following completion of management for underlying cardiovascular or pulmonary diseases and at least two weeks after improvement of cholangitis and restoration of liver function tests).
With respect to procedure-related complications, the incidences of pancreatitis, cholangitis, bleeding, or malfunction in our study was similar to incidences reported by others (26-28). There were no significant differences in procedure-related complication rates between the two groups, with the exception of procedure-related pancreatitis: the pancreatitis rate was higher in the endoscopic BD group than in the PTBD group. Two of the pancreatitis cases in the endoscopic BD group prolonged the postponement of surgery because medical management of the pancreatitis was needed. Such a postponement would be unjustifiable for a potentially resectable tumor.
Cost-effectiveness was evaluated by comparing total hospital cost, admission cost, and hospital stay duration. There were no significant differences between the two groups on the basis of the final drainage method used; however, on an intention-to-treat basis, admission cost was lower in the PTBD group than in the endoscopic BD group. This could be related to those patients who were initially subjected to endoscopic BD and who were changed to PTBD. There were no significant difference in postoperative complication rate between two groups, but the cost for managing postoperative complications in the intentioned endoscopic BD group was higher than in the intentioned PTBD group. This is in contrast with an earlier randomized study in which van der Gaag et al. (29) looked at 202 patients with cancer of the pancreatic head. In their multicenter, randomized trial they found no effect of preoperative BD, primarily attempted endoscopically, on the length of the hospital stay. They explained that the previously observed extended hospital stay durations can be attributed to the use of PTBD, whereas endoscopic BD, as used primarily in their study, is often performed on an outpatient basis. In our study, the hospital stay was comparable between PTBD and endoscopic BD.
There are reports of metastatic tumor seeding along the PTBD sinus tract (28, 30). Takahashi et al. (28) retrospectively investigated 445 patients with perihilar and distal cholangiocarcinoma who underwent resection following PTBD and detected PTBD catheter tract recurrence in 23 of those patients. They also reported poor prognosis in PTBD catheter tract recurrence patients. Moreover, they supported the association of PTBD with serious complications, such as vascular injury and cancer dissemination, and suggested that ENBD is the most suitable method for initial preoperative BD. In this study, among 6 PTBD patients with a bleeding complication, 3 patients underwent gelfoam embolization without further complication, and there was no tract recurrence until the last follow up (median follow up of 23 months, range 0.2-46 months). In contrast with their study, our study included periampullary lesions but excluded hilar cholangiocarcinomas. In Klatskin tumor cases, we believe it unlikely that, passing through the tumor during the procedure is not needed and can be succeed safely in periampullary cancer. However, the only way to answer this question adequately would be to conduct a large prospective, randomized trial.
In conclusion, using an intention-to-treat analysis, the PTBD group revealed a more rapid serum bilirubin decline with a shorter drainage duration and a lower admission cost compared with the endoscopic BD group. Although the role of preoperative BD is still controversial, preoperative BD is needed in some situations. The results in this study indicate that PTBD is more cost-effective and safer than endoscopic BD. Practitioners should consider those factors in selecting a BD method.
Figures and Tables
Fig. 1
A graph of bilirubin decline pattern in the endoscopic BD groups. ERBD, endoscopic retrograde biliary drainage; ENBD, endoscopic naso-biliary drainage.

Table 2
Comparision of clinicopathologic characteristics according to final biliary drainage methods
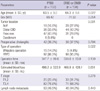
Table 3
Comparision of cilinicopathologic characteristics according to biliary drainage methods after intention-to-treat analysis
References
1. Wang Q, Gurusamy KS, Lin H, Xie X, Wang C. Preoperative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev. 2008. CD005444.
2. Pitt HA, Gomes AS, Lois JF, Mann LL, Deutsch LS, Longmire WP Jr. Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost? Ann Surg. 1985. 201:545–553.
3. Speer AG, Cotton PB, Russell RC, Mason RR, Hatfield AR, Leung JW, MacRae KD, Houghton J, Lennon CA. Randomized trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet. 1987. 2:57–62.
4. Povoski SP, Karpeh MS Jr, Conlon KC, Blumgart LH, Brennan MF. Association of preoperative biliary drainage with postoperative outcome following pancreaticoduodenectomy. Ann Surg. 1999. 230:131–142.
5. van der Gaag NA, Kloek JJ, de Castro SM, Busch OR, van Gulik TM, Gouma DJ. Preoperative biliary drainage in patients with obstructive jaundice: history and current status. J Gastrointest Surg. 2009. 13:814–820.
6. Kimmings AN, van Deventer SJ, Obertop H, Rauws EA, Huibregtse K, Gouma DJ. Endotoxin, cytokines, and endotoxin binding proteins in obstructive jaundice and after preoperative biliary drainage. Gut. 2000. 46:725–731.
7. Sewnath ME, Karsten TM, Prins MH, Rauws EJ, Obertop H, Gouma DJ. A metaanalysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg. 2002. 236:17–27.
8. Sewnath ME, Birjmohun RS, Rauws EA, Huibregtse K, Obertop H, Gouma DJ. The effect of preoperative biliary drainage on postoperative complications after pancreaticoduodenectomy. J Am Coll Surg. 2001. 192:726–734.
9. Roebuck DJ, Stanley P. External and internal-external biliary drainage in children with malignant obstructive jaundice. Pediatr Radiol. 2000. 30:659–664.
10. Baijal SS, Dhiman RK, Gupta S, Sharma BC, Roy S, Agarwal DK, Choudhuri G, Saraswat VA, Naik SR. Percutaneous transhepatic biliary drainage in the management of obstructive jaundice. Trop Gastroenterol. 1997. 18:167–171.
11. Kawarada Y, Higashiguchi T, Yokoi H, Vaidya P, Mizumoto R. Preoperative biliary drainage in obstructive jaundice. Hepatogastroenterology. 1995. 42:300–307.
12. Joseph PK, Bizer LS, Sprayregen SS, Gliedman ML. Percutaneous transhepatic biliary drainage. Results and complications in 81 patients. JAMA. 1986. 255:2763–2767.
13. Karsten TM, Davids PH, van Gulik TM, Bosma A, Tytgat GN, Klopper PJ, van der Hyde MN. Effects of biliary endoprostheses on the extrahepatic bile ducts in relation to subsequent operation of the biliary tract. J Am Coll Surg. 1994. 178:343–352.
14. Hatfield AR, Murray RS. Pre-operative biliary drainage in patients with obstructive jaundice. A comparison of the percutaneous transhepatic and endoscopic transpapillary routes. S Afr Med J. 1981. 60:737–742.
15. Speer AG, Cotton PB, Russell RC, Mason RR, Hatfield AR, Leung JW, MacRae KD, Houghton J, Lennon CA. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet. 1987. 2:57–62.
16. Gouma DJ, van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, Busch OR, Obertop H. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000. 232:786–795.
17. Huibregtse K. Complications of endoscopic sphincterotomy and their prevention. N Engl J Med. 1996. 335:961–963.
18. van Berge Henegouwen MI, van Gulik TM, Akkermans LM, Jansen JB, Gouma DJ. The effect of octreotide on gastric emptying at a dosage used to prevent complications after pancreatic surgery: a randomised, placebo controlled study in volunteers. Gut. 1997. 41:758–762.
19. Kimmings AN, Van Deventer SJ, Obertop H, Rauws EA, Huibregtse K, Gouma DJ. Endotoxin, cytokines, and endotoxin binding proteins in obstructive jaundice and after preoperative biliary drainage. Gut. 2000. 46:725–731.
20. Hatfield AR, Tobias R, Terblanche J, Girdwood AH, Fataar S, Harries-Jones R, Kernoff L, Marks IN. Preoperative external biliary drainage in obstructive jaundice. A prospective controlled clinical trial. Lancet. 1982. 2:896–899.
21. Lai EC, Mok FP, Fan ST, Lo CM, Chu KM, Liu CL, Wong J. Preoperative endoscopic drainage for malignant obstructive jaundice. Br J Surg. 1994. 81:1195–1198.
22. Hwang DW, Kim SW, Yoon YS, Kim JH, Jang JY, Park YH. Preoperative biliary drainage for periampullary cancer: a comparison between endoscopic drainage and percutaneous transhepatic drainage. J Korean Surg Soc. 2003. 65:413–419.
23. Fan ST, Lo CM, Lai EC, Yu WC, Wong J. T-lymphocyte function in patients with malignant biliary obstruction. J Gastroenterol Hepatol. 1994. 9:391–395.
24. Hunt DR, Allison ME, Prentice CR, Blumgart LH. Endotoxemia, disturbance of coagulation, and obstructive jaundice. Am J Surg. 1982. 144:325–329.
25. Hunt CE, Diani AR, Brown PK, Kaluzny MA, Epps DE. Diet induced atherogenic hyperlipoproteinaemia and liver injury in cynomolgus macaques. Br J Exp Pathol. 1986. 67:235–249.
26. Christensen M, Matzen P, Schulze S, Rosenberg J. Complications of ERCP: a prospective study. Gastrointest Endosc. 2004. 60:721–731.
27. Williams EJ, Taylor S, Fairclough P, Hamlyn A, Logan RF, Martin D, Riley SA, Veitch P, Wilkinson ML, Williamson PR, Lombard M. Risk factors for complication following ERCP: results of a large-scale, prospective multicenter study. Endoscopy. 2007. 39:793–801.
28. Takahashi Y, Nagino M, Nishio H, Ebata T, Igami T, Nimura Y. Percutaneous transhepatic biliary drainage catheter tract recurrence in cholangiocarcinoma. Br J Surg. 2010. 97:1860–1866.
29. van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ, Gerritsen JJ, Greve JW, Gerhards MF, de Hingh IH, Klinkenbijl JH, Nio CY, de Castro SM, Busch OR, van Gulik TM, Bossuyt PM, Gouma DJ. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010. 362:129–137.
30. Chapman WC, Sharp KW, Weaver F, Sawyers JL. Tumor seeding from percutaneous biliary catheters. Ann Surg. 1989. 209:708–713.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download